Clinical utility of EUS-guided sampling of solid pancreatic lesions: diagnostic spectrum, treatment strategies, and prognostic insights from a single center
Tomáš Koller1, Petra Vrbová1, Gabriel Holst1, Dana Dudová1, Iveta Mečiarová2, Viliam Gál2, Miroslav Tomáš3, Jozef Dolník3
+ Affiliation
Summary
Background: Solid pancreatic lesions (SPLs) pose significant diagnostic and therapeutic challenges due to their high malignant potential and limited treatment options. Endoscopic ultrasound (EUS) -guided tissue sampling has become a cornerstone in their evaluation, offering minimally invasive access for histological confirmation. This study aimed to assess the clinical utility of EUS-guided biopsy for SPLs in a single center. Methods: We retrospectively analyzed patients who underwent EUS-guided tissue sampling for SPLs at the University Hospital Bratislava between 2018 and 2023. Exclusion criteria included cystic lesions or negative EUS findings. Data on demographics, lesion characteristics, sampling techniques, pathology, treatment modalities, and survival outcomes were collected. Diagnostic categories were stratified, and survival analyses were performed using Kaplan-Meier curves and Cox regression modeling. Results: Out of 747 EUS procedures, 159 met the inclusion criteria. The median patient age was 67 years; 62.3% were male. The most frequent diag- nosis was pancreatic adenocarcinoma (61.6%), followed by unspecified malignant lesions (10.1%), benign lesions (8.2%), and neuroendocrine tumors (7.5%). Malignant or potentially malignant lesions accounted for 83.9% of cases. EUS-guided biopsy was diagnostic in 90.6% of cases with no major complications recorded. Treatment (surgery, chemotherapy, or both) was administered to 48.4% of the patients. Surgical intervention and chemotherapy were both associated with significantly improved survival, with the best outcomes observed in patients receiving combined therapy. Surgery was the only independent predictor of reduced mortality (HR = 0.376; P = 0.002). Conclusion: EUS-guided sampling is a safe and effective tool for diagnosing SPLs, with high diagnostic yield and direct impact on treatment planning. Integration of histological findings into multidisciplinary management significantly influences prognosis, particularly in patients eligible for surgical resection and/or chemotherapy. These findings underscore the pivotal role of EUS within a coordinated care framework for SPLs.
Keywords
endosonography, pancreatic neoplasms, biopsy fine-needle, retrospective studies, survival analysis, adenocarcinoma, multidisciplinary communication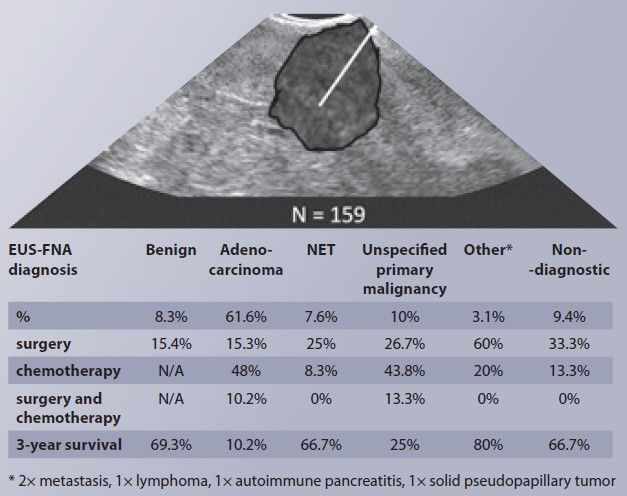
Introduction
Focal pancreatic lesions are common findings in imaging studies. Their diagnosis and management remain challenging, particularly because solid pancreatic lesions often carry a relatively high malignant potential. At the same time, pancreatic resection, the only potentially curative option, is not suitable for all patients. Early identification of malignant potential is therefore essential for appropriate management [1]. Cross-sectional imaging techniques demonstrate high accuracy in lesion detection; however, their ability to reliably distinguish between benign and malignant lesions remains suboptimal [2]. Currently, endoscopic ultrasonography (EUS) with targeted biopsy represents the most effective modality for obtaining tissue samples in a minimally invasive manner [3,4]. Once a definitive diagnosis is established, diagnostic findings must be integrated with the clinical context through a multidisciplinary consensus to formulate an optimal treatment plan and assess prognosis [5].
The spectrum of patients referred for EUS-guided biopsy varies depending on the institutional context and specialization. In addition to the technical quality of EUS, the expertise of the entire clinical team and practical implementation of multidisciplinary recommendations are key determinants of a center’s overall effectiveness [6]. For these reasons, the clinical impact of EUS-guided sampling for solid pancreatic lesions is unique to each institution and reflects its role within the broader continuum of care. We hypothesize that a retrospective single-center analysis of EUS performance could offer valuable insights into its role in the current management of these complex cases.
Our study aimed to retrospectively evaluate the clinical utility of endosonographic tissue sampling of solid pancreatic lesions at a single center. Specifically, we sought to:
1. Analyze the diagnostic outcomes obtained from EUS-guided tissue sampling.
2. Assess the treatment modalities administered based on multidisciplinary decision-making.
3. Evaluate patient prognosis in relation to the histopathological types of solid pancreatic lesions and the corresponding therapeutic approaches.
Methods
This retrospective study included patients who underwent endoscopic ultrasound (EUS) with targeted tissue sampling at the Gastroenterology and Hepatology Division of the 5th Department of Internal Medicine, University Hospital Bratislava, due to a suspected solid pancreatic lesion identified on prior imaging (computed tomography, magnetic resonance imaging, or transabdominal ultrasonography). Patients in whom only a cystic lesion was identified during EUS, or in whom no pancreatic lesion was confirmed, were excluded from the analysis. The inclusion period spanned six years, from 2018 to 2023. The following parameters were recorded: age, sex, date of EUS examination, referring department, lesion size (if delineated), type and gauge of the needle, number of passes, serum CA19-9 levels (if available), EUS findings, and results of cytological and histological analyses (see below). Follow-up data included subsequent treatment modalities, specifically: surgery (with timing and type), chemotherapy (with timing, regimen, and duration), neoadjuvant chemotherapy (with timing and duration), and radiotherapy. Survival data were obtained from the national registry of insured persons, with follow-up continued until September 13, 2024. Follow-up was censored at the date of death or the end of the observation period for surviving patients. All patients signed an informed consent form before any procedure, and data capture has been approved by the local ethics committee.
EUS procedures were performed using the Fujifilm 530UT or 580UT systems under local anesthesia, with continuous oxygen saturation monitoring. Tissue sampling was conducted using either fine-needle aspiration (FNA, Expect) or fine-needle biopsy (FNB, Acquire) needles (Boston Scientific), with gauges of 22G or 25G. The standard sampling procedure was as follows: a suspicious pancreatic lesion was identified using linear EUS. A pre-rinsed thin needle (without stylet) was introduced into the working channel, attached to a syringe preset with a 10 mL vacuum and a closed valve. Under EUS guidance, the needle was inserted into the lesion, the syringe valve was opened, and 5–10 passes were made through the lesion using the fanning technique. The syringe valve was then closed, and the needle withdrawn. Material was expressed onto a slide using a stylet and positive pressure from the syringe. Macroscopic tissue fragments (visible cores) were identified and transferred to a vial containing 4% formalin for histopathological analysis. Residual material was smeared onto a second slide and left to air dry. This process was repeated 1- to 4-times, depending on the apparent adequacy of the collected material.
Cytological findings were classified according to the Papanicolaou Society of Cytopathology system (categories I–VI). Histological samples were fixed, stained with hematoxylin and eosin, and if required, supplemented with additional immunohistochemical or special stains at the discretion of the pathologist. Final diagnoses were established by integrating cytological and histological findings with clinical and imaging data. For statistical purposes, diagnoses were grouped into five categories: Pancreatic adenocarcinoma, Unspecified malignant pancreatic lesion, Neuroendocrine tumor (NET), Benign lesion, and Undiagnosed lesion. Finally, each case was reviewed by the referring institution‘s multidisciplinary team to determine further management.
Statistical analysis was performed using MedCalc® software v. 23.2.6 (MedCalc Software Ltd., Ostend, Belgium; https: //www.medcalc.org; 2025) and R (The R Project for Statistical Computing, https: //www.r-project.org/) with the EZR package for R Commander (Y. Kanda). Data are presented as medians with interquartile ranges or as absolute numbers and percentages. A P-value < 0.05 was considered statistically significant. Continuous variables were compared using the Mann-Whitney test; categorical variables were compared using the Chi-square test. Kaplan-Meier survival curves were generated based on diagnostic categories. Independent predictors of mortality were identified using the Cox proportional hazards regression analysis.
Results
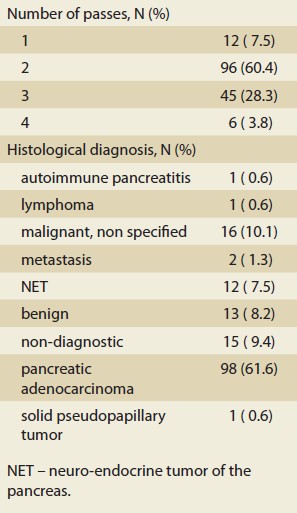
During the study period, we performed 747 EUS examinations, of which 232 were EUS-guided samplings. About 159 procedures in 155 patients met the inclusion and exclusion criteria. The basic characteristics of the group are shown in Tab. 1. The median age was 67 years, and 62.3% of them were men. The most common localization of the lesion was in the head of the pancreas in 64.2% of cases, followed by the body in 14.5% and the tail in 5.7% of cases. In 23 (16.7%) cases, the lesion extended into two areas of the pancreas. The median lesion size was 27 mm. Up to 88% of patients had a fine-needle biopsy (FNB) compared to 12% who had fine-needle aspiration (FNA). In 80%, we used a 22G needle, and in 60.4% of cases, they had two passes through the lesion.
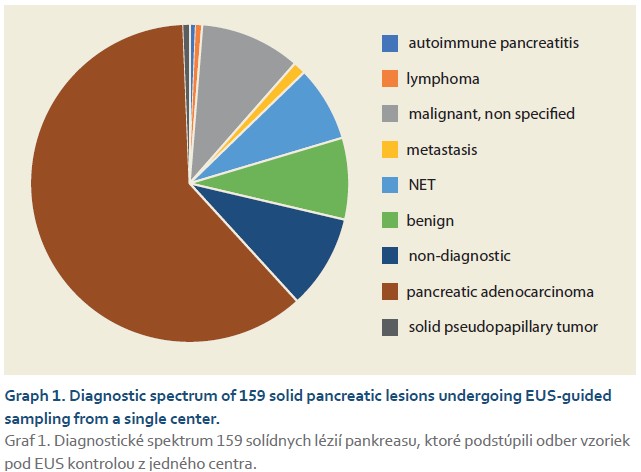
The diagnostic spectrum of the solid lesions is displayed in Graph 1. The most frequently diagnosed lesion was pancreatic adenocarcinoma in 98 (61.6%) cases, followed by an unspecified malignant lesion of the pancreas in 16 (10.1%) cases, a benign lesion in 13 (8.2%) cases, and a neuroendocrine tumor in 12 (7.5%) cases. Rarer lesions were autoimmune pancreatitis, lymphoma, metastasis, or a solid pseudopapillary tumor. Out of a total of 155 patients, we diagnosed a lesion with malignant potential (adenocarcinoma, unspecified malignant lesion, NET, lymphoma, metastasis, pseudopapillary tumor) in 130 (83.9%) patients. In 15 (9.4%) cases, the lesion could not be diagnosed further even by cytological or histological examination.
During the observation period, we did not record any serious complications of EUS-guided samplings that would be associated with the need for hospitalization, extension of hospitalization, or other intervention such as transfusions.
In Tab. 2, we compared the basic characteristics and treatment modalities of the five diagnostic groups. Out of a total of 155 patients, 75 (48.4%) underwent a treatment modality based on the result. In Tab. 2, we present the spectrum of treatment modalities that patients underwent based on the decision of the multidisciplinary teams. Finally, the table also shows prognostic data on patient survival after 1, 2, and 3 years.
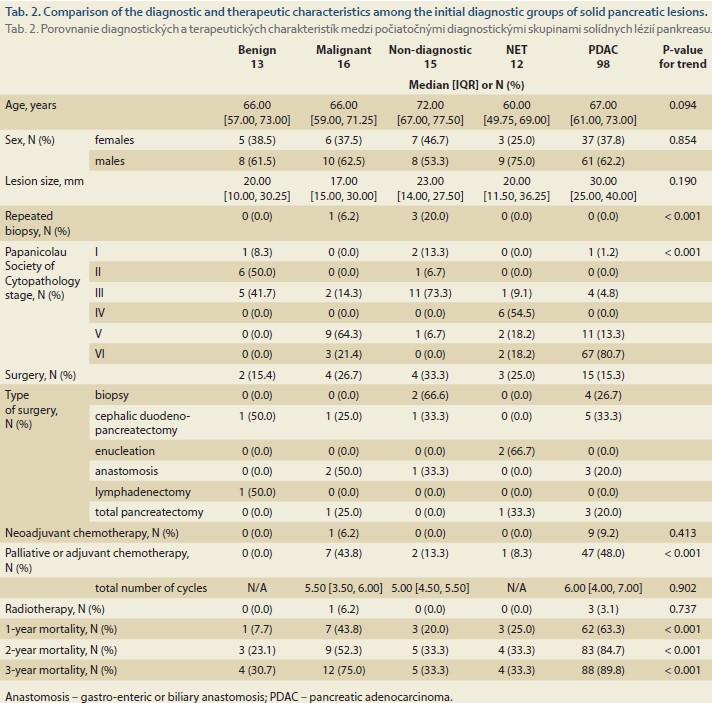
Benign lesions
In 13 cases with a median age of 66 years, we found a benign lesion, of which four patients had signs of chronic pancreatitis in their history, and another two had an atrophic gland in the EUS image. Out of this group, only one patient underwent resection for suspected symptomatic paraduodenal pancreatitis, and one patient underwent lymphadenectomy. Mortality within 1 and 3 years in this group of patients was 7.7% and 30.7% respectively.
Nondiagnostic sampling
In 15 cases with nondiagnostic sampling, the median age was 72 years. In three cases, patients underwent repeat biopsy. In one patient, the second biopsy revealed a benign lesion; in two of them, the second biopsy confirmed adenocarcinoma. Four other patients underwent surgical treatment, one of them had resection and chemotherapy, one had biopsy and subsequent chemotherapy, one had biopsy without further treatment, and one had anastomosis. The remaining eight patients from this group did not undergo any treatment modality, while their mortality within 1 and 3 years was 0% and 25.0% respectively.
Neuroendocrine tumor
A group of 12 patients with neuroendocrine tumors and with a median age of 60 years was predominantly male (75%). Three underwent surgical resection, one underwent chemotherapy, while the remaining lesions were sent in for follow-up. Mortality within 1 and 3 years was 25% and 33.3% respectively.
Malignant lesions
In a group of 16 patients, the samplings detected pancreatic malignancy without further specification. As part of the differential diagnosis, the pathologist formulated the possibilities such as ductal or acinar adenocarcinoma, NET, or metastasis. One patient had a repeat biopsy, which, however, did not provide a more detailed diagnosis. The lesions were the smallest in comparison with the other groups, with a median of 17 mm. Two patients underwent resection surgery, two had anastomotic surgery, and seven patients (43.8%) had chemotherapy. Mortality within 1 and 3 years was 43.8% and 75% respectively.
Pancreatic adenocarcinoma
The most numerous group was pancreatic adenocarcinoma in 98 cases (62%) with a median age of 67 years, and also with a predominance of men (62%). In this group, the median size of the solid lesion was the highest (30 mm). Out of these, eight patients (8.1%) had subsequent resection surgery, four underwent additional biopsies by surgical route (4%), and three underwent a surgical biliary or gastro-enteric anastomosis. A total of 9 patients (9.2%) underwent neoadjuvant chemotherapy, and 47 (48%) had adjuvant or palliative chemotherapy with a median of six cycles. Three patients (3%) underwent radiotherapy. Mortality within 1 and 3 years was 63.3% and 89.8% respectively.
The lymphoma patient underwent successful chemotherapy, the SPT patient underwent successful resection surgery, and the autoimmune pancreatitis patient underwent successful immunosuppressive therapy. One patient with metastatic renal cell carcinoma underwent pancreatic resection, and one patient with metastatic lung cancer underwent anastomotic surgery.
Prognostic insights
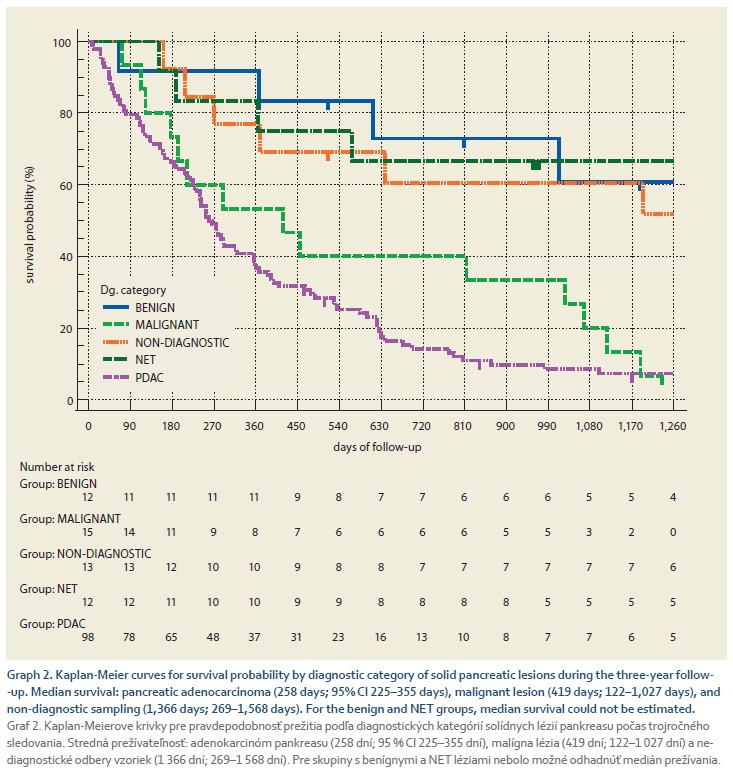
Graph 2 displays the survival probability by diagnostic category of solid pancreatic lesions during the three-year follow-up. The median survival was lowest for pancreatic adenocarcinoma (258 days, 95% CI 225–355 days), a malignant lesion (419 days, 122–1027 days), and an undiagnosed lesion (1366 days, 269-1568 days). In the other groups, the probability of survival during the follow-up period did not fall below 50%.
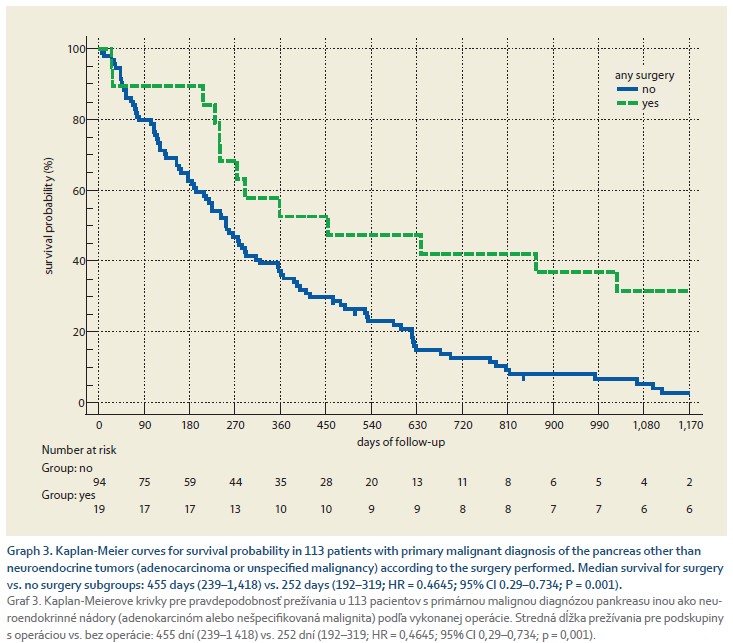
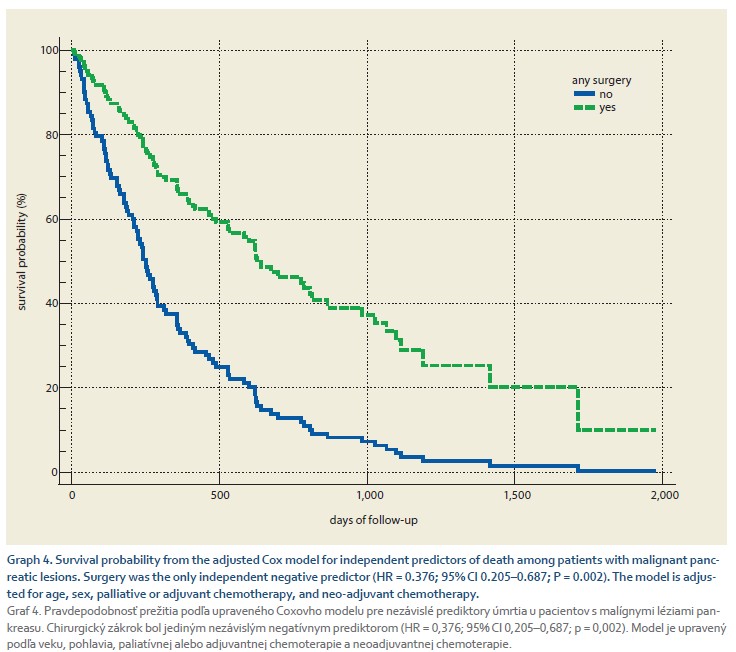
Graph 3 shows a comparison of the survival probability in 113 patients with a primary malignant diagnosis of the pancreas other than neuroendocrine tumors (adenocarcinoma or unspecified malignancy) according to the surgery performed. Any surgery performed in 19/113 (16.8%) patients with a median delay of 60 days (95% CI 20.4–144.9) days was associated with a significant improvement in survival with a median of 455 days (239–1,418) vs. 252 days (192–319, HR = 0.4645; 95% CI 0.29–0.734; P = 0.001) in those who did not undergo surgery. We modeled factors independently associated with death using a Cox model. We included age, sex, any surgery, palliative or adjuvant chemotherapy, and neo-adjuvant chemotherapy in the model. We found that surgery was the only independent negative predictor of death (HR = 0.376; 95% CI 0.205–0.687; P = 0.002). The survival curve according to surgery performed, adjusted for all factors, is shown in Graph 4.
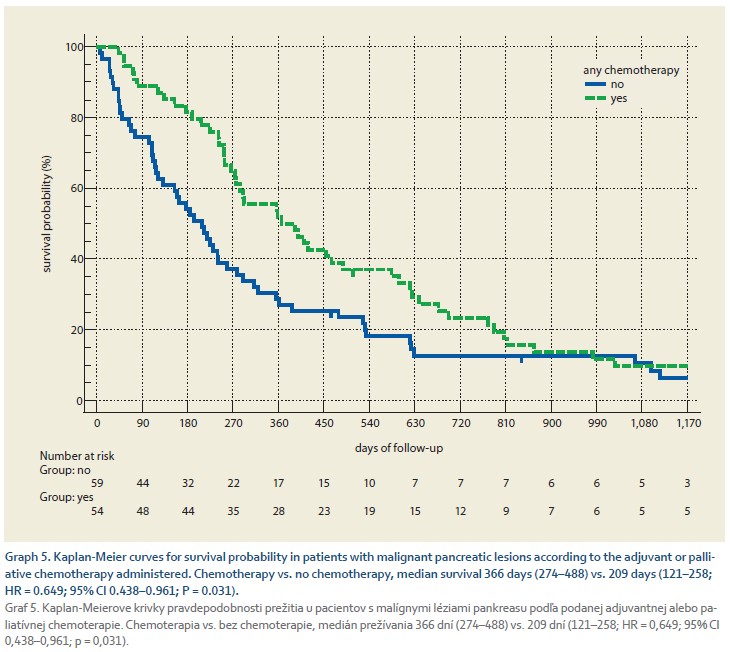
Graph 5 shows the survival probability of 113 patients with pancreatic cancer according to the adjuvant or palliative chemotherapy administered. Chemotherapy was administered to 55 (48.7%) patients with a median delay of 35 days (95% CI 25.7–43.0), which was associated with significant improvement in survival with a median of 366 days (274–488) compared to 209 days (121–258, HR = 0.649; 95% CI 0.438–0.961; P = 0.031) in those who did not receive chemotherapy.
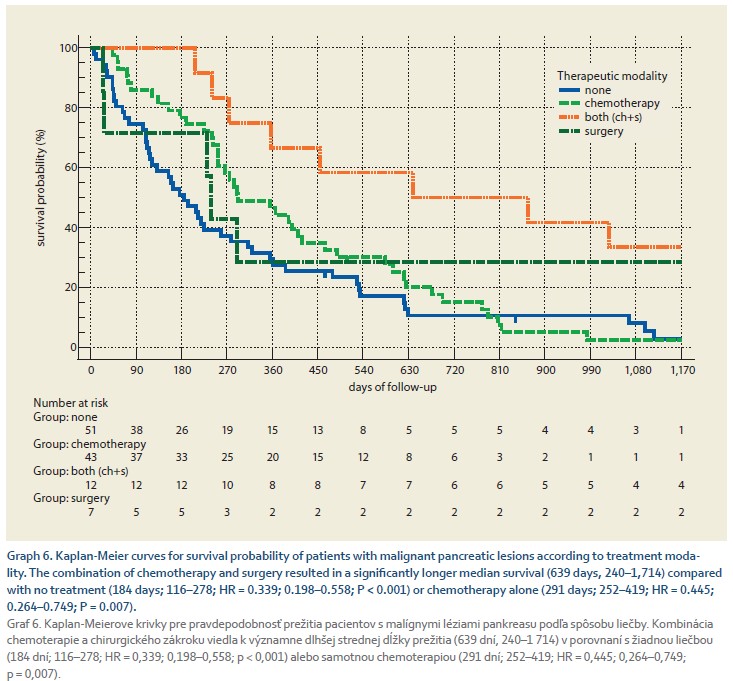
Graph 6 shows the survival probability of 113 patients with pancreatic cancer according to treatment modality. Out of these, 51 (45%) had no treatment, 43 (38%) patients received chemotherapy, 7 (6.2%) received surgery alone, and 12 (10.6%) patients received a combination of chemotherapy and surgery. The combination of chemotherapy and surgery resulted in significantly longer median survival (639 days, 240–1714) compared with no treatment (184 days, 116–278, HR = 0.339; 0.198–0.558; P < 0.001) or chemotherapy alone (291 days, 252–419, HR = 0.445; 0.264–0.749; P = 0.007).
Discussion
In our study, we retrospectively analyzed the clinical impact of EUS-guided biopsy of 159 solid pancreatic lesions in 155 patients. This modern tissue sampling technique provided diagnostic certainty in approximately 90% of cases. In over 80% of cases, the lesions were identified as having malignant potential, while approximately 10% were benign. Another 10% remained non-diagnostic after the first sampling. Tissue-based diagnosis of solid pancreatic lesions proved to be a key prognostic factor. The diagnosis allowed for curative or palliative treatment strategies in nearly 50% of cases, following multidisciplinary consultation. Treatment modalities had a favorable impact on survival in pancreatic malignancies, with the combination of surgery and chemotherapy demonstrating the most benefit.
Our observed rates of malignant lesions and non-diagnostic biopsies were consistent with data from the literature and meta-analyses [7]. In our practice, we primarily used fine-needle biopsy (88%), which became available in our country during the study period. This approach aligns with current ESGE guidelines for the biopsy of solid pancreatic lesions [8]. Technically, we used two passes in most cases (60%), which is consistent with findings from a recent meta-analysis [9], suggesting that two passes provide an optimal diagnostic yield. Due to the absence of an on-site histopathologist during EUS procedures and our evolving experience with macroscopic on-site evaluation (MOSE), the number of passes was individually adapted based on immediate macroscopic assessment. Recent studies suggest that factors such as lesion size on EUS and cellularity, which cannot be fully assessed during the procedure, play a crucial role in determining tissue adequacy for molecular analysis [10].
In approximately 10% of cases, sampling was non-diagnostic. These lesions had a mean size of 23 mm and could be divided into two typical clinical scenarios. In the first one, imaging suggested malignancy (e. g., blurred margins, upstream pancreatic duct dilatation, vascular invasion, lymphadenopathy), and a non-diagnostic sampling could not rule out cancer. In such cases, repeat sampling either via EUS or surgery was recommended and performed in 7 patients. In the second scenario, imaging and EUS showed no clear signs of malignancy. Here, the interpretation of the non-diagnostic result reduced suspicion of malignancy. Multidisciplinary teams did not recommend further diagnostic steps in eight patients. This subgroup (median age 72 years) had 0% one-year and 25% three-year mortality. Due to the retrospective design, the exact cause of death in these patients could not be determined.
Benign lesions were found in around 10% of cases, consistent with literature data indicating that 4–11% of solid pancreatic lesions are benign and do not require specific treatment [11,12]. The most common benign entities include chronic pancreatitis, autoimmune pancreatitis [13,14], or intrapancreatic accessory spleen [15]. Several patients in this group had features suggestive of chronic or paraduodenal pancreatitis or glandular atrophy. EUS-guided biopsy was useful in these cases for two main reasons. First, it potentially prevented unnecessary pancreatic resections, which based on literature estimates, occur in 5–11% of cases when malignancy is incorrectly assumed [12,16,17]. Risk factors for such misdiagnoses include abdominal pain and alcohol abuse, whereas malignancy is more often associated with jaundice and ductal dilatation. Second, a negative biopsy result can significantly improve the quality of life for patients with suspected malignancy [18]. However, cases have been reported where malignancy (e. g., adenocarcinoma) was found in resected specimens despite a negative preoperative EUS biopsy. These patients often had chronic pancreatitis features, ductal dilatation, or a history of pancreatic surgery [19]. In our cohort, one patient underwent resection and another underwent lymphadenectomy, though definitive histology was unavailable. These findings highlight the complexity of differentiating malignancy from chronic pancreatitis, even with modern diagnostic tools [20].
More than 80% of patients had malignant pancreatic lesions. Excluding those with clear curative intent (e. g., lymphoma, solid pseudopapillary tumor, metastases, neuroendocrine tumors), we focused on a subgroup of 113 patients with primary pancreatic malignancies (adenocarcinoma or undifferentiated carcinoma). Out of these, 62 (55%) underwent surgical and/or systemic treatment. Our data show that treatment significantly improved survival (Graphs 3–6). The median interval from biopsy to treatment was 60 days for surgery and 35 days for chemotherapy. Although literature recommends initiating chemotherapy within 4 weeks and continuing for 6 months, this was not always feasible in our real-world setting [21].
The remaining 51 patients with malignant lesions received no oncological or surgical treatment. A slightly lower percentage of this clinical outcome has been reported from a single center in our region [22]. While the diagnostic benefit of EUS-guided biopsy in this group may seem limited, the literature identifies several reasons for non-treatment: patient refusal, poor performance status (ECOG > 2 or Karnofsky < 70%), comorbidities, or advanced disease stage [23]. Although we could not assess specific causes due to the retrospective design, establishing a tissue-based diagnosis could still aid in prognosis and facilitate timely palliative care, such as EUS-guided celiac plexus neurolysis, nutritional support, hospice referral, and psychosocial planning. From an ethical standpoint, diagnostic confirmation of malignancy offers limited clinical benefits if it is known in advance that the patient will not undergo any treatment. In such cases, forgoing EUS-guided biopsy may be a reasonable option [24].
Our study has several limitations. Its retrospective nature limited our ability to assess detailed clinical data, including exact TNM staging, comorbidities, and patient preferences. Because our study only included patients undergoing EUS--guided biopsy, it did not represent the full spectrum of pancreatic malignancies and was not designed to compare treatment modalities. Surgical procedures were performed across multiple centers, so detailed histopathological data were not always available. Nonetheless, the study had several strengths. All EUS procedures were performed by a single medical team, histopathological analyses were conducted in a centralized pathology department by two experienced specialists, and survival data were verified through a national registry.
Conclusion
In our clinical setting, EUS-guided tissue sampling of solid pancreatic lesions proved to have substantial clinical utility. It provided sufficient material for accurate pathological diagnosis and yielded high diagnostic accuracy with a strong prognostic value. When biopsy results were promptly integrated into multidisciplinary treatment decisions, even patients with advanced pancreatic malignancy experienced improved survival. However, accurate diagnosis and smooth implementation of multidisciplinary recommendations remain challenging in the context of chronic pancreatitis and/or limited resources. In patients with poor performance status or advanced disease, where biopsy results are unlikely to influence management, the decision to forego EUS-guided sampling may be ethically and clinically justified.
ORCID authors
T. Koller 0000-0001-7418-0073,
P. Vrbová 0000-0003-4881-8787,
G. Holst 0009-0006-9056-0045,
D. Dudová 0009-0009-6996-2922,
I. Mečiarová 0000-0002-7385-335X,
V. Gál 0000-0001-9435-7825,
M. Tomáš 0000-0002-1114-5120,
J. Dolník 0009-0002-4694-5045.
Submitted/Doručené: 30. 7. 2025
Accepted/Prijaté: 20. 8. 2025
Corresponding author
Assoc. Prof. Tomáš Koller, MD, PhD
Gastroenterology and Hepatology Division
5th Department of Internal Medicine
Faculty of Medicine, Comenius University University Hospital Bratislava
Ružinovská 6
826 06 Bratislava
tomas.koller@fmed.uniba.sk
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Iglesias-Garcia J, De La Iglesia-Garcia D, Olmos-Martinez JM et al. Differential diagnosis of solid pancreatic masses. Minerva Gastroenterol Dietol 2020; 66(1): 70–81. doi: 10.23736/S1121- 421X.20.02646-X.
2. Pulkertová A, Špičák J, Gábriš V et al. Correlation of indications for pancreatic resection with histopathological findings. Gastroenterol Hepatol 2012; 66(3): 191–195.
3. Machicado JD, Sheth SG, Chalhoub JM et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the diagnosis and management of solid pancreatic masses: summary and recommendations. Gastrointest Endosc 2024; 100(5): 786–796. doi: 10.1016/j.gie.2024.06.002.
4. Cazacu IM, Chavez AA, Saftoiu A et al. A quarter century of EUS-FNA: progress, milestones, and future directions. Endosc Ultrasound 2018; 7(3): 141–160. doi: 10.4103/eus.eus_19_18.
5. Francisse S, Gkolfakis P, Viesca MFY et al. The impact of a multidisciplinary team approach on the management of focal pancreatic lesions: a single tertiary center experience. Ann Gastroenterol 2023; 36(5): 580–587. doi: 10.20524/aog.2023.0827.
6. Schutz HM, Quispel R, Veldt BJ et al. Cumulative sum learning curves guiding multicenter multidisciplinary quality improvement of EUS-guided tissue acquisition of solid pancreatic lesions. Endosc Int Open 2022; 10(4): E549–E557. doi: 10.1055/a-1766-5259.
7. Hewitt MJ, McPhail MJ, Possamai L et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012; 75(2): 319–331. doi: 10.1016/j.gie.2011.08.049.
8. Facciorusso A, Arvanitakis M, Crinò SF et al. Endoscopic ultrasound-guided tissue samling: European Society of Gastrointestinal Endoscopy (ESGE) technical and technology review. Endoscopy 2025; 57(4): 390–418. doi: 10.1055/a-2524-2596.
9. Chalhoub JM, Hawa F, Grantham T et al. Effect of the number of passes on diagnostic performance of EUS fine-needle biopsy of solid pancreatic masses: a systematic review and meta-analysis. Gastrointest Endosc 2024; 100(4): 595.e8–604.e8. doi: 10.1016/j.gie.2024.05.022.
10. Mohamed WT, Jahagirdar V, Jaber F et al. Endoscopic ultrasound-guided fine-needle biopsy versus aspiration for tissue sampling adequacy for molecular testing in pancreatic ductal adenocarcinoma. Cancers (Basel) 2024; 16(4): 761. doi: 10.3390/cancers16040761.
11. Misra S, Mahajan V, Kansal S et al. Benign pathologies encountered in the whipple pancreatico-duodenectomy specimen – 11-year experience from a tertiary care center. Int J Surg Pathol 2025; 33(6): 1411–1424. doi: 10.1177/10668969251323932.
12. Yarandi SS, Runge T, Wang L et al. In- creased incidence of benign pancreatic pathology following pancreaticoduodenectomy for presumed malignancy over 10 years despite increased use of endoscopic ultrasound. Diagn Ther Endosc 2014; 2014: 701535. doi: 10.1155/2014/701535.
13. Van Heerde MJ, Biermann K, Zondervan PE et al. Prevalence of autoimmune pancreatitis and other benign disorders in pancreatoduodenectomy for presumed malignancy of the pancreatic head. Dig Dis Sci 2012; 57(9): 2458–2465. doi: 10.1007/s10620-012-2191-7.
14. Dite P, Novotny I, Dvorackova J et al. Pancreatic solid focal lesions: differential diagnosis between autoimmune pancreatitis and pancreatic cancer. Dig Dis 2019; 37(5): 416–421. doi: 10.1159/000499762.
15. Shon F, Štiková Z, Maxa V et al. Intrapancreatic accessory spleen as a rare solid pancreatic lesion. Gastroenterol Hepatol 2018; 72(6): 522–526.
16. Gerritsen A, Molenaar IQ, Bollen TL et al. Preoperative characteristics of patients with presumed pancreatic cancer but ultimately benign disease: a multicenter series of 344 pancreatoduodenectomies. Ann Surg Oncol 2014; 21(12): 3999–4006. doi: 10.1245/s10434-014-3810-7.
17. Surci N, Rösch CS, Kirchweger P et al. The rate of avoidable pancreatic resections at a high-volume center: an internal quality control and critical review. J Clin Med 2023; 12(4): 1625. doi: 10.3390/jcm12041625.
18. Cazacu IM, Chavez AA, Mendoza TR et al. Quality of life impact of EUS in patients at risk for developing pancreatic cancer. Endosc Ultrasound 2020; 9(1): 53–58. doi: 10.4103/eus.eus_56_19.
19. Davidyuk V, Bhutiani N, Gold MK et al. Surgical diagnoses of pancreatic adenocarcinoma not found on previous endoscopic ultrasound: a case series and review of the literature. Am Surg 2023; 89(4): 990–995. doi: 10.1177/0003 1348211054531.
20. Löhr JM, Vujasinovic M, Maisonneuve P. Risk of pancreatic cancer in inflammatory pancreatic diseases. Korean J Gastroenterol 2025; 85(3): 280–285. doi: 10.4166/kjg.2025.019.
21. Okada K, Uemura K, Sumiyoshi T et al. Survival benefit of adjuvant therapy completion with early initiation for patients with pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg 2024; 9(4): 785–793. doi: 10.1002/ags3.12898.
22. Kliment M, Urban O, Loveček M et al. Endosonography-guided fine needle aspiration biopsy of solid pancreatic masses – accuracy and impact on the treatment of 358 patients. Gastroenterol Hepatol 2013; 67(5): 431–437.
23. Sundaresan TK, Evans NS, Gupta S et al. Treatment rates and reasons for non-treatment in a real-world cohort of patients with pancreatic cancer. J Clin Oncol 2024; 42(Suppl 16): e13554. doi: 10.1200/JCO.2024.42.16_suppl.e13554.
24. Fusaroli P, Rakichevikj E, Lisotti A. Quality in EUS: for many but not for all. Best Pract Res Clin Gastroenterol 2025; 76: 102010. doi: 10.1016/j.bpg.2025.102010.