Trichohepatoenteric syndrome in a patient with TTC37 mutations – a case report
Petr Jabandžiev1, Eva Hlaváčková2, Viktor Bílý2, Eva Karásková3, Lumír Kunovský4,5, Hana Grombiříková2, Romana Kozumplíková6, Hana Bučková1, Kateřina Slabá1, Tereza Pinkasová1, Martin Jouza1, Jakub Pecl1, Marta Ježová4, Václava Curtisová7, Tomáš Freiberger1, Barbora Ravčuková Orcid.org 8
+ Affiliation
Summary
We report a patient with somatic retardation and woolly hair appearance who suffered from recurring episodes of watery mucous diarrhea, impaired liver functions, and failure to thrive. He manifested with severe infection courses, including hepatitis of unknown origin complicated by liver failure at 4 months, bronchopneumonia at 4 years, and life-threatening sepsis with septic shock at 8 years of age. Esophagogastroduodenoscopy and colonoscopy were performed at 4 years to rule out inflammatory bowel disease (IBD), and only signs of nonspecific colitis were evident. Immunology workup revealed slight reduction in CD4+ naive subsets and impaired T cell response to mitogens. Massive parallel sequencing (also termed next-generation sequencing – NGS) targeting a panel of primary immunodeficiency-related genes was used to examine the patient’s DNA. NGS analysis revealed two heterozygous variants in the TTC37 gene. Nonsense p.Arg1201* and missense p.Leu1505Ser variants in exons 34 and 42, respectively, were evaluated as pathogenic based on in silico predictions, their rare occurrence in the general population, and the fact that both mutations had already been described in patients with trichohepatoenteric syndrome (THES). As clinical features in our patient were in accordance with this diagnosis, we consider our findings as causative. THES could be a life-threatening condition, particularly in children who develop liver disease or severe infection courses. THES can have a similar clinical presentation as does very early-onset inflammatory bowel disease (VEO-IBD) and is often assigned to this group. Although IBD is generally regarded as a polygenic disease, some children with VEO-IBD are known also to have diseases with monogenic etiologies, as in THES. Targeted NGS is an efficient tool for establishing an accurate dia gnosis in VEO-IBD patients.
Keywords
trichohepatoenteric syndrome, very early-onset inflammatory bowel disease, children, next-generation sequencingIntroduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder the incidence of which has been increa sing rapidly in the past decade, and especially in child populations [1,2]. Very early-onset IBD (VEO-IBD) comprises a group of children who are diagnosed before 6 years of age and includes a diverse spectrum of rare genetic disorders. Some of these monogenic disorders do not respond to conventional therapy and demand effective targeted therapies due to their association with high morbidity and mortality. Because these diseases are rare and can manifest with heterogeneous phenotypes, a correct diagnosis is often delayed [3]. The key component of diagnosis is a combination of clinical history, evaluation of immune functions, and molecular genetic analysis using targeted sequencing pa nels or whole-exome sequencing (WES). Sequencing today plays an increasingly important diagnostic role. A large number of immunodeficiencies and epithelial cell defects can be associated with VEO-IBD and severe phenotypes. Early identification of VEO-IBD with a genetic basis and, in particular, immunodeficiencies is crucial for assessing prognosis and targeting therapy to the dysfunctional pathway [4,5].
Case report
We present the case report of a 9-year- -old boy with chronic watery stools with mucus, failure to thrive, growth impairment, and medical history of several severe infection courses at the time of diagnosis.
This first child from a first, uncomplicated pregnancy was born in the 40th week of gestation to Caucasian nonconsanguineous parents. Delivery was spontaneous, birth weight was 3,000 grams, birth length was 49 cm, and the Apgar score was of no physiological concern (10-10-10). The patient was fully breastfed, and total time of breastfeeding was 4 months. At 4 months of age, he was admitted to the hospital with hepatitis of unknown origin complicated by liver failure. The patient had an episode of rotavirus gastroenteritis at 2 years of age. Streptococcus pneumoniae pneumonia manifested at 4 years of age. Failure to thrive and frequent chronic watery stools with mucus and without blood was evident from the second year of life. Esophagogastroduodenoscopy and colonoscopy were carried out at the age of 4 years without signs of macroscopic pathology. Histologically, only nodular hyperplasia of the terminal ileum was confirmed, and signs of nonspecific colitis were evident. Due to the patient’s persistent clinical symptoms, it was decided to perform a control colonoscopy at 8 years of age. During a routine stan dard bowel emptying before examination, the patient developed septic shock with pleuropneumonia leading to lung failure with the need for artificial ventilation. Throughout the follow-up, the patient received no specific therapy other than symptomatic.
The patient’s appearance was marked by a short, asthenic stature, tiny face, and brittle blond hair (Fig. 1). There was no skin or joint pathology present. Mental impairment was not recognized. Because there were signs of gastrointestinal tract impairment and medical history of severe infection courses, an inborn error of immunity (IEI) was suspected. Complement or phagocytoses deficiency was ruled out. The patient revealed no laboratory sign of antibody deficiency, and therefore cellular deficiency was suspected. Slight IgA hypergammaglobulinemia without any sign of IgG or IgM hypogammaglobulinemia was present. Routine post-vaccination antibody levels of IgG against pneumococcal capsular polysaccharide, tetanus toxoid, and Haemophilus influenzae type b antigen were protective. Polysaccharide antigen response was not evaluated. The absolute counts and percentage numbers of T CD3+CD4+, NK CD16/56+, and B CD19+ lymphocyte subsets remained within the reference range. Slight T CD3+CD8+ lymphocytosis and mild reduction of CD3+CD4+ naive subsets were present. T cell lymphoproliferative response to mitogens was impaired (ConA, PHE, antiCD3antiCD28). Interpretation of decreased T cell receptor excision circles (TRECs) and kappa-deleting recombination excision circles (KRECs) could be questionable at this age.
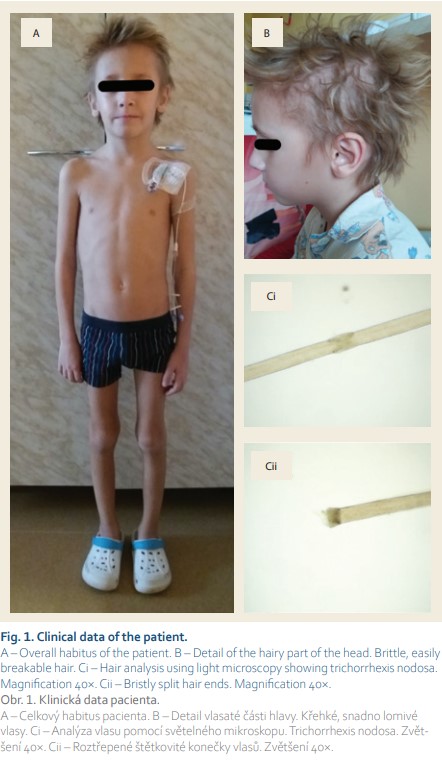
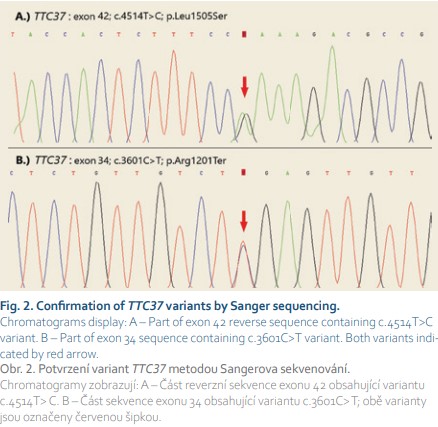
Next-generation sequencing (NGS) targeting a panel of primary immunodeficiency-related genes was used to examine the patient’s DNA. The NGS analysis revealed two heterozygous variants in the TTC37 gene (Fig. 2). No additional relevant pathogenic or likely pathogenic mutations were identified by NGS. Both nonsense p.Arg1201* and missense p.Leu1505Ser variants in exons 34 and 42, respectively, were evaluated as pathogenic based on in silico predictions and/or their rare occurrence in the general population. Considering the facts that both mutations had already been described in patients with trichohepatoenteric syndrome (THES) and that clinical features in our patient were in accordance with this diagnosis, we considered our finding as causative, even though it could not be confirmed that both alleles were affected due to the impossibility to investigate the DNA of the patient’s parents.
From the age of 8 years, the patient was stable without manifestation of any severe infectious course with persistent diarrhea. Sadly, the patient died tragically at age 10 for reasons unrelated to this main diagnosis.
Discussion
THES is a rare autosomal recessive syndromic enteropathy with a recently described molecular basis. To date, only about 100 affected individuals have been reported in the literature [6]. THES is a potentially life-threatening pediatric condition, particularly in children with liver disease or severe infection courses. Our patient also went through several life-threatening events, although his death in a car accident was not related to this condition. THES is generally consi dered to be a neonatal enteropathy and is characterized by intractable diarrhea (seen in almost all affected children), woolly hair (seen in all), intrauterine growth restriction, facial dysmorphism, and short stature. Additional findings include recurrent or severe infections, skin abnormalities, and liver disease. Mild intellectual disability is seen in about 50% of affected individuals. Less common findings include congenital heart defects and platelet anomalies [6–8]. Sim larly to VEO-IBD, THES is characterized as a combined immunodeficiency with associated syndromic features according to the 2019 International Union of Immunological Societies (IUIS) updated phenotypical classification [9]. Immunology workup in such cases could reveal hypogammaglobulinemia (IgG, IgA), IgA monoclonal gammopathy, impaired post-vaccination response, and/or impaired interferon gamma (IFNγ) production [6,9].
THES is caused by mutation of either TTC37 or SKIV2L, two genes that encode two components of the human SKI complex [6,8,10]. The SKI complex is a tetraprotein complex including an RNA helicase subunit encoded by SKIV2L, a subunit made of a tetratrico peptide repeats (TPR) protein encoded by TTC37, and two subunits of a WD40 (Trp/Asp repeats) domain containing protein encoded by WDR61. The SKI complex is the cofactor of the RNA exosome and is involved in such specific functions of the RNA exosome as cytoplasmic mRNA degradation, particularly in case of STOP loss and/or gain and post-endoribonucleolytic cleavage residues decay. The SKI complex is also known to play a role in antiviral responses [6,11].
To date, THES is the only known Mendelian disorder associated with these two TTC37 and SKIV2L genes. It is inherited as an autosomal recessive trait, with a penetrance of 100%. With the exceptions of a few patients evidencing only one mutation, only strict homozygous or compound heterozygous cases have been reported, and digenic inheri tance has never been observed. Gene rally, THES is characterized by the association of nine main clinical signs: intractable diarrhea, hair abnormalities, facial dysmorphism, intrauterine growth restriction (IUGR), immunodeficiency, skin abnormalities, liver disease, congenital cardiac defects, and platelet anomalies. Bourgeois et al stated that patients mutated in SKIV2L seem to be more severely affected than are patients with TTC37 mutations, with seve ral signs being noticed earlier in life or more serious. In fact, 37% of TTC37 defective patients have been observed to have cardiac defects, 39% skin abnormalities, 51% liver disease, 56% immune defect, 70% IUGR, 84% facial dysmorphia, 95% hair abnormalities, and 100% diarrhea [6,7]. Clinical features of our patient were in full accordance with the diagnosis of THES (liver disease, immune defect, facial dysmorphia, hair abnormalities, and diarrhea).
Very-early-onset IBD
THES can have a clinical presentation similar to that of VEO-IBD and is often assigned to this group. Evaluating and managing children with VEO-IBD is a challenging field for pediatric gastroenterologists worldwide [12]. The incidence of childhood IBD is increasing and constitutes 8–25% of all IBD cases, with 6–15% presenting under the age of 6 years [1–3,13,14]. Childhood IBD is understood as IBD onset at younger than 17 years of age, where IBD diagnosed before 6 years of age constitutes VEO-IBD. Infantile IBD sets before 2 years of age, and neonatal IBD manifests before 28 days of life [14].
IBD in general is regarded as a polygenic disease, and a genome-wide association study has revealed more than 230 known disease-associated genes. However, some children with VEO-IBD are known also to have diseases with monogenic etiologies. Recent advances in genetic evaluation have enabled the identification of gene mutations responsible for some forms of IBD. Monogenic or Mendelian disorder-associated IBD (MD-IBD) is a term used to represent IBD caused by genetic mutations [12].
Inborn errors of immunity
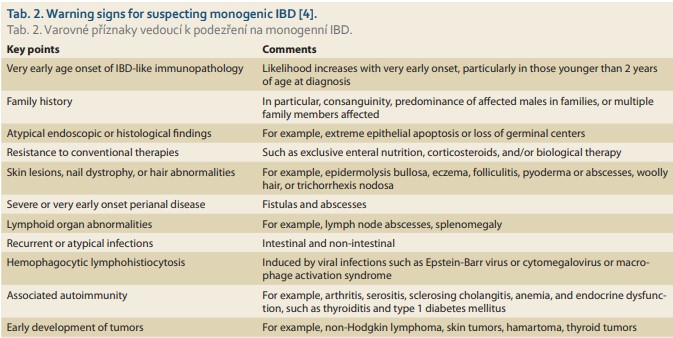
A subset of more aggressive, therapy-resistant VEO-IBD with early onset and frequently with positive family history is recently understood to be associated with inborn errors of immunity (IEI) [3]. The updated list of IEI from the IUIS phenotypical classification comprises 406 IEI disorders with 430 gene defects [9]. More than 50 IBD or IBD-like associated monogenic defects have been identified [15]. VEO-IBD related to IEI manifests due to impairment of intestinal epithelial barrier function, defects in phagocyte bacterial killing, increased hyper- or autoimmune inflammatory pathways, or impaired development and function of the adaptive immune system (Tab. 1). A gastrointestinal manifestation of IEI is common and may precede the principal IEI diagnosis [3,15]. Failure to thrive and diarrhea could be the first signs of IEI in general. Consanguinity, positive family history (IBD vs. IEI), multiple miscarriages, personal history of severe infectious disease courses, opportunistic infection, fungal infection or recurrent or persistent viral infection (e. g., Epstein--Barr virus, cytomegalovirus, papillomavirus, herpes simplex virus), recurrent deep skin or internal organ abscesses, mucocutaneous candidiasis, recurrent sinopulmonary infection, autoimmunity (type 1 diabetes mellitus, various forms of cytopenia, autoimmune endocrinopathy, etc.), benign lymphoproliferation with or without hepatosplenomegaly, and failure to thrive constitute the main IEI warning signs [14,16]. Also, skin lesions, malignancy, or onset of hemophagocytic lymphohistiocytosis in an IBD patient refractory to conventional therapy raises suspicion of monogenic IBD origin [15].

Impairment of the intestinal barrier epithelial surface leads to a transepithelial transformation of commensal bacteria, inflammation, and dysbio sis increasing the inflammation loop [17]. Chronic granulomatosis disease with impaired NADPH oxidase function could mimic IBD [13]. VEO-IBD is understood as the main sign of genetic variants in the IL-10-IL-10R pathway and related cytokine family member disorders [8].
Underlying immune dysregulation dominates in a group of genetic variants impairing T regulatory cell (Treg) function. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX) should always be considered if IBD is accompanied by type I diabetes mellitus manifesting during the first year of life. IPEX-like disorders include disturbed IL2-IL2R interaction, signal transducer and activator of transcription 5b (STAT5b) deficiency, lipopolysaccharide-responsive beige-like anchor protein (LRBA) deficiency, and cytotoxic T lymphocyte antigen 4 (CTLA4) deficiency. Complex central immune dysregulation represents autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy (APECED) [9,13].
A broad group of genetic variants impairing development of the adaptive immune system consist of severe combined immunodeficiency (SCID), inclu ding Omenn syndrome, combined immunodeficiencies with syndromic fea - tures (Wiskott-Aldrich syndrome, THES), and predominantly antibody deficiencies (common variable immunodeficiency – CVID) [3,13].
IBD could also be a sign of autoinflammatory disorders, such as mevalonate-kinase deficiency (hyper IgD syndrome, HIDS), familial Mediterranean fever, or NLRC4-associated inflammatory disease [13].
Clinical warning signs leading to suspicion of IBD-like or monogenic VEO-IBD are summarized in Tab. 2. Leukopenia, lymphocytopenia, and neutropenia should be ruled out in the immunology workup [16]. Autoimmune cytopenias could signal underlying immune dysregulation. To exclude antibody deficiency or combined immunodeficiency, IgG, IgM, and IgA levels and/or IgG and IgA subclasses compared with age-adjusted levels should be evaluated [16,19]. Hyper IgM syndromes should be consi dered if IgG and IgA are decreased or absent but IgM is elevated or normal [20]. Elevated IgE could be together with other characteristic features a sign of hyper IgE syndromes. Decreased post-vaccination titers or impaired response to polysaccharide and/or protein antigen is a routine diagnostic tool in antibody deficiencies settings [16,19]. Flow cytometry phenotyping of main T CD3+, CD3+CD4+, CD3+CD8+, B CD19+, and NK CD16/56 lymphocyte subsets have diagnostic and prognostic value [20]. Impaired T or B cell lymphoprolife rative response to mitogens, reduced naive T cell, reduced recent thymic immigrants, and reduced or absent TRECs underline cellular or combined immunodeficiency [20,21]. Dihydrorhodamine burst test must be evaluated when chronic granulomatous disease is suspected. In cases of potential hemophagocytic lymphohistiocytosis, NK cell cytotoxicity tests are part of a diagnostic algorithm [9]. Flow cytometry protein expression (LRBA, CTLA4), protein signaling (STAT3), and interleukin expression (IL10) help in confirming diagnoses [20].
Next-generation sequencing
Whole-exome sequencing or IBD sequencing panels provide diagnostic assurance [13]. Massive parallel sequen cing, also termed next-generation sequencing (NGS), is a technology for detecting gene variants and enables us to analyze hundreds and thousands of genes, or even an entire genome, in a short period of time. The capacity of NGS offers new opportunities for clinical application and is increasingly used for establishing disease diagnosis and prognosis and in making therapeutic decisions [22].
The most commonly used NGS assay is targeted sequencing, which typically interrogates tens or hundreds of genes presumed to be associated with a particular clinical phenotype or group of diseases (i.e., primary immunodeficiencies). Targeted NGS panels are gaining popularity due to their time- and cost-effectiveness. A specific type of targeted NGS is an assay analyzing all protein-coding regions of the genome. Known as whole-exome sequencing (WES), it is particularly beneficial in cases of disorders that are phenotypically heterogeneous and in which it is difficult to select an appropriate panel of candidate causative genes. Despite its apparent advantages, however, WES also has some limitations, such as that intronic and noncoding regions remain uncovered or that sequencing quality (often expressed as sequencing depth) is insufficient for particular genes, simply because the range of targeted regions is too large. Finally, it should be said that NGS technology produces huge amounts of data such that their processing and storage presents a great bio informatic challenge [23].
When properly selected and used, NGS technologies constitute an excellent tool that greatly expands the range of genes analyzed and can contribute importantly to ensuring adequate preventive and therapeutic procedures for at-risk patients [22].
Conclusion
VEO-IBD is often a severe and debilita ting form of IBD and frequently overlaps with immune deficiencies and other rare diseases. The majority of IBD are thought to be polygenic, but rare cases can be attributed to disease-causing variants within a single gene, including TTC37 in the case of THES. Targeted NGS is an efficient tool for establishing an accurate diagnosis in VEO-IBD or VEO-IBD-like patients.
Submitted/Doručeno: 30. 9. 2020
Accepted/Přijato: 15. 11. 2020
Prof. Tomáš Freiberger, MD, PhD
Centre for Cardiovascular Surgery and Transplantation
Výstavní 17/19
603 00 Brno
Czech Republic
tomas.freiberger@cktch.cz
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Jabandziev P, Pinkasova T, Kunovsky L et al. Regional incidence of inflammatory bowel disease in a czech pediatric population: 16 years of experience (2002–2017). J Pediatr Gastroenterol Nutr 2020; 70 (5): 586–592. doi: 10.1097/MPG.0000000000002660.
2. Sýkora J, Pomahačová R, Kreslová M et al. Cur rent global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol 2018; 24 (25): 2741–2763. doi: 10.3748/wjg.v24.i25.2741.
3. Kelsen JR, Russo P, Sullivan KE. Early-onset inflammatory bowel disease. Immunol Allergy Clin North Am 2019; 39 (1): 63–79. doi: 10.1016/j.iac.2018.08.008.
4. Uhlig HH, Schwerd T, Koletzko S et al. The dia gnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014; 147 (5): 990–1007. e3. doi: 10.1053/j.gastro.2014.07.023.
5. Ouahed J, Spencer E, Kotlarz D et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis 2020; 26 (6): 820–842. doi: 10.1093/ibd/izz259.
6. Bourgeois P, Esteve C, Chaix C et al. Tricho-Hepato-Enteric Syndrome mutation update: Mutations spectrum of TTC37 and SKIV2L, clinical analysis and future prospects. Hum Mutat 2018; 39 (6): 774–789. doi: 10.1002/humu.23418.
7. Fabre A, Bourgeois P, Chaix C et al. Trichohepatoenteric Syndrome. In: Adam MP, Ardinger HH, Pagon RA et al (eds). GeneReviews®. Washington: University of Washington 2018.
8. Fabre A, Charroux B, Martinez-Vinson C et al. SKIV2L mutations cause syndromic diarrhea, or trichohepatoenteric syndrome. Am J Hum Genet 2012; 90 (4): 689–692. doi: 10.1016/j.ajhg.2012.02.009.
9. Bousfiha A, Jeddane L, Picard C et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical cl assification. J Clin Immunol 2020; 40 (1): 66–81. doi: 10.1007/s10875-020-00758-x.
10. Hartley JL, Zachos NC, Dawood B et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy). Gastroenterology 2010; 138 (7): 2388–2398: e1–e2. doi: 10.1053/j.gastro.2010.02.010.
11. Schaeffer D, Clark A, Klauer AA et al. Functions of the cytoplasmic exosome. Adv Exp Med Biol 2011; 702: 79–90. doi: 10.1007/ 978-1-4419-7841-7_7.
12. Arai K. Very early-onset inflammatory bowel disease: a challenging field for pediatric gastroenterologists. Pediatr Gastroenterol Hepatol Nutr 2020; 23 (5): 411–422. doi: 10.5223/pghn.2020.23.5.411.
13. Kelsen JR, Sullivan KE, Rabizadeh S et al. NASPGHAN position paper on the evaluation and management for patients with very early-onset inflammatory bowel disease (VEO-IBD). J Pediatr Gastroenterol Nutr 2020; 70 (3): 389–403. doi: 10.1097/MPG.0000000000002567.
14. Nameirakpam J, Rikhi R, Rawat SS et al. Genetics on early onset inflammatory bowel dis ease: an update. Genes Dis. 2020; 7 (1): 93–106. doi: 10.1016/j.gendis.2019.10. 003.
15. Shim JO. Recent advance in very early onset inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutr 2019; 22 (1): 41–49. doi: 10.5223/pghn.2019.22.1.41.
16. de Vries E, members ESID. Patient-centred screening for primary immunodeficiency, a multi-stage dia gnostic protocol designed for non-immunologists: 2011 update. Clin Exp Immunol 2012; 167 (1): 108–119. doi: 10.1111/j.1365-2249.2011.04461.x.
17. Tegtmeyer D, Seidl M, Gerner P et al. Inflammatory bowel disease caused by primary immunodeficiencies-Clinical presentations, review of literature, and proposal of a rational dia gnostic algorithm. Pediatr Allergy Immunol 2017; 28 (5): 412–429. doi: 10.1111/pai.12734.
18. Zhu L, Shi T, Zhong C et al. IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterology Res 2017; 10 (2): 65–69. doi: 10.14740/gr740w.
19. Wood P, Stanworth S, Burton J et al. Recognition, clinical dia gnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol 2007; 149 (3): 410–423. doi: 10.1111/j.1365-2249.2007. 03432.x.
20. Abraham RS, Aubert G. Flow cytometry, a versatile tool for dia gnosis and monitoring of primary immunodeficiencies. Clin Vaccine Immunol 2016; 23 (4): 254–271. doi: 10.1128/CVI.00001-16.
21. Kalina T, Bakardjieva M, Blom M et al. EuroFlow standardized approach to dia gnostic immunopheneotyping of severe PID in newborns and young children. Front Immunol 2020; 11: 371. doi: 10.3389/fimmu.2020.00371.
22. Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 2016; 17 (6): 333–351. doi: 10.1038/nrg. 2016.49.
23. Slatko BE, Gardner AF, Ausubel FM. Overview of next-generation sequencing technologies. Curr Protoc Mol Biol 2018; 122 (1): e59. doi: 10.1002/cpmb.59.