Cystic fibrosis and exocrine pancreatic insufficiency
Pavla Tesaříková1,2, Lumír Kunovský3,4, Jan Trna Orcid.org 5, Petr Dítě Orcid.org 1,6, Petr Jabandžiev7, Jitka Vaculová8, Zdeněk Kala Orcid.org 3
+ Affiliation
Summary
Cystic fibrosis (CF) is a genetic disease affecting many organs, including the gastrointestinal tract. While the pulmonary damage is the most life threatening, the pancreas is one of the first organs affected by CF and one of the most strongly affected. Mutation in the CF transmembrane conductance regulator (CFTR) gene results in a reduced volume of pancreatic juice and hyperconcentration of macromolecules leading to precipitation in the duct lumina, causing obstruction and damage. The clinical presentation of individual cases depends on a combination of different CFTR mutations, the potential presence of modifier gene mutations and environmental factors. CFTR mutations are historically divided into 5 classes – severe mutations (classes 1–3) and mild mutations (classes 4–5). The CFTR functional status depends on the combined effects of both CFTR allels and the severity of the phenotype depends on the milder mutation. The majority of CF patients exhibit exocrine pancreatic insufficiency in early childhood because functional acinar tissue has been lost in utero or soon after birth. These patients rarely suffer from pancreatic complications such as recurrent acute pancreatitis and/or chronic pancreatitis which, however, can occur in the minority of patients who possess residual pancreatic exocrine function. CFTR mutations are found more frequently in idiopathic and alcoholic chronic pancreatitis but the data is conflicting. A combination with serine protease inhibitor Kazal-type 1 (SPINK-1) mutations can be found in the idiopathic chronic pancreatitis group, as well as the presence of environmental factors. Malnutrition is directly related to a worse prognosis of CF patients and the delivery of active digestive enzymes is a cornerstone of treatment, with acid supression and vitamin supplementation playing an important additional role.
Keywords
CFTR, cystic fibrosis, exocrine pancreatic insufficiency, malnutrition, pancreatitisIntroduction
Cystic fibrosis (CF) is the most common fatal genetic defect with autosomal recessive heredity, which is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [1]. The overall prevalence among the neonate cohort in the CZ between 2010 and 2017 was 1: 6,536 [2]. The Czech CF patient registry currently maintains data on almost 900 CF patients (> 700 living, almost 50% older than 18 years of age) with an annual acquisition of about 20 newborn CF patients [3].
The CFTR gene encodes the CFTR protein that functions primarily as a transmembrane chloride channel. It is also highly expressed in the pancreatic duct epithelia where it conducts anions (bicarbonates in particular) and water to the ductal lumen and thus allows the highly concentrated proteins secreted by the acinar cells to stay in a soluble state. Therefore, a reduced or absent CFTR channel function results in a reduced volume of pancreatic juice and hyperconcentration of macromolecules leading to precipitation in the duct lumina, causing obstruction and damage [4]. Reduction of bicarbonate conductance due to unfavourable CFTR conformation limits the flush of pancreatic enzymes from the ducts and puts the pancreas at risk of either recurrent acute pancreatitis (RAP) or progressive organ destruction because of premature activation [5]. Blockage of the pancreatic ductules leads to necrosis of the ductal and centroacinar cells, inspisation of secretion and eventually to the destruction of the acini, causing flattening and atrophy of the epithelium. These changes lead to gradual atrophy as well as cystic and fibrotic destruction of the pancreas. This process can sometimes be documented through imaging [6]. Histology findings show end-stage chronic pancreatitis (CP) features and dilated ducts appearing as multiple protein-filled cysts are typical.
Screening of cystic fibrosis
CF is a life-threatening disease for which early diagnosis following newborn screening improves the prognosis. Since 2009, a two-step nationwide screening program of CF is conducted in the CZ [7]. In the first step, a blood sample is taken 72–96 hours after birth and dried blood spots are examined for immunoreactive trypsinogen (IRT) concentration. In the second step, newborns with elevated IRT are tested for CFTR mutations. Newborns with elevated IRT and proven CFTR mutation undergo a classic sweat test. If the results of the aforementioned tests are indeterminate, transepithelial potential difference in the nasal or rectal mucosa can be performed [8].
Genetic counselling in families with a positive family history of CF (including the parents of newborn CF patients) has been a standard of care since the 1980s. If conception among positive cases is planned, the utilisation of prenatal diagnostics and/or assisted reproduction can lead to a decrease in CF incidence.
Testing of the pancreatic exocrine function is an integral part of the initial examination of a newly diagnosed CF patient. However, the currently available diagnostic tools are mostly imperfect or complicated to perform. The coefficient of fat absorption is considered the gold standard by some, but it is cumbersome to perform. Faecal elastase is the most widely used test, since it is a simple and relatively reliable marker from 2 weeks of age and in the absence of liquid stools. Pancreatic sufficient patients should be monitored by annual faecal elastase during infancy and childhood and during periods of failure to thrive, weight loss or diarrhoea [9]. Other pancreatic function tests described in literature have not reached wide clinical use [10].
Clinical presentation
CF is characterised by wide clinical variability, including progressive lung disease, pancreatic dysfunction, elevated sweat electrolytes and male infertility. Expected survival for typical forms of CF have improved significantly over the last century with the current median survival above 40 years of age [11]. While the pulmonary damage is the most life threatening, the early clinical features of CF are gastrointestinal symptoms such as maldigestion of either pancreatic or intestinal origin. The pancreas is one of the organs affected first and most strongly by CF. Almost 90% of CF patients present with exocrine pancreatic insufficiency and CFTR gene mutations constitute the vast majority (about 85%) of children with pancreatic insuficiency [12].
Clinical symptoms can be less pronounced in children than in adults. Exocrine pancreatic insufficiency should be considered in children who fail to thrive, in the presence of chronic diarrhoea, abdominal distension, symptoms of small intestinal bacterial overgrowth, unexplained oedemas and/or laboratory abnormalities such as hypoalbuminaemia, anaemia and fat-soluble vitamin or trace element deficiencies [9,10]. Physicians should also focus on the possible presence of other organ symptoms specific for each disease causing exocrine pancreatic insufficiency – CF, Shwachmann-Diamond syndrome, Johanson-Blizzard syndrome, Pearson syndrome, Jeune syndrome [13].
Pathophysiology
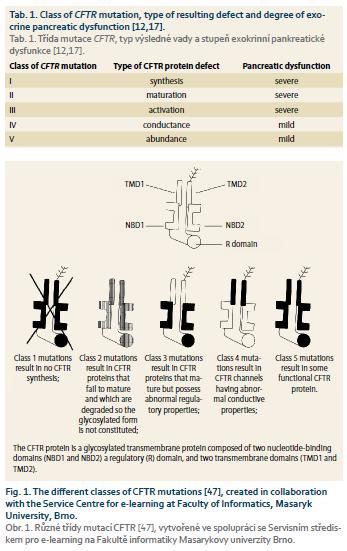
The extent of pancreatic disease among individuals with CFTR mutations varies. The clinical presentation of individual cases depends on a combination of different CFTR mutations, the potential presence of modifier gene mutations and environmental factors [12]. There have been almost 2,000 different mutations of the CFTR gene described, although about 20 are found the most frequently [14]. The mutations are historically divided into 5 classes (with a sixth class proposed, but not generally accepted) according to the type of defect caused to the CFTR protein and according to the severity of the clinical phenotype [15–17]. Details are provided in Tab. 1 and Fig. 1. The CFTR functional consequence depends on the combined effects of both CFTR allels and the severity of the phenotype depends on the milder mutation [18,19]. Severe mutations (classes 1–3) lead to no functional protein and are associated with severe pancreatic dysfunction when combined with a second severe CFTR mutation. Mild mutations (classes 4–5) result in CFTR protein with altered but residual function and lead to milder pancreatic dysfunction. There is a good correlation between the genotype-phenotype and pancreatic involvement [20]. Two severe mutations produce pancreatic insufficiency while one or two mild mutations lead to preserved pancreatic functions in most cases. In general, a combination of mutations from mild and severe classes result in milder CF with only a subset of affected organs [21]. This situation is sometimes called atypical CF. If a severe mutation (class 1–3) is combined with wild-type (heathy) CFTR or a benign polymorphism, then CFTR function is reduced by up to 50%, which results in a normal phenotype since a 90% reduction must occur before clinical signs are present. RAP and CP are also linked to these heterozygous or polymorphic CFTR genotypes [22]. However, parents of CF patients (who are CFTR mutation carriers without CF) do not show increased incidence of RAP or CP when compared with the normal population and therefore, an additional pancreas affecting factor is presumed necessary [23]. This factor may be the coinciding serine protease inhibitor Kazal-type 1 (SPINK-1) mutation, other complex genetic alterations, potent environmental factors (smoking, alcohol) and/or the presence of pancreas divisum [24–28].
From a clinical perspective, CF patients can be divided into pancreatic sufficient (PS) and pancreatic insufficient (PI). While the majority of CF patients are PI, up to 15% possess sufficient pancreatic exocrine function to permit normal digestion [29]. RAP or CP develops in less than 2% of CF patients, appearing in adolescence or young adulthood, and almost exclusively among PS patients (usually those patients with one severe and one mild CF mutation) [30]. The reason may be that this genetic combination leads to more impaired chloride transport than bicarbonate secretion, resulting in less effective pancreatic functions [31]. Conversely, several specific mutations exist that predominantly affect bicarbonate secretion. These mutations may cause pancreas-specific injury or may be at least a part of a more complex trait of CP. The patients affected by them are at risk of isolated pancreatic disease (RAP or CP) without affecting the organs that use CFTR primarily to transport chloride [32]. PI patients are usually free of these complications because functional acinar tissue has been lost in utero or soon after birth. However, exceptions to this rule can occur – a large multicentre study with a follow-up of a total of 10,071 patients with CF from 29 different countries described the occurrence of pancreatitis in 0.5% of PI patients. Nevertheless, this was significantly lower than the occurence of pancreatitis in 10.27% of PS patients [30].
CFTR in idiopathic and alcoholic chronic pancreatitis
Conflicting results in the prevalence of CFTR mutations among patients with idiopathic and alcoholic CP have been published. Some researchers find CFTR mutations more frequently in idiopathic and alcoholic CP when compared to controls but other studies do not confirm this finding [33,34]. One study found that about 20% of patients with idiopathic CP carried at least one severe CFTR mutation, while in the control group only 3–4% of individuals carry one CFTR-causing mutation. About 15% of patients with idiopathic CP were found to be compound heterozygous for two mutations, one of them being mild. Some of these patients may even show a positive sweat test, but generally without pulmonary CF symptoms. A combination with SPINK-1 mutations can be found in the idiopathic CP group as well [35].
Treatment of cystic fibrosis-related exocrine pancreatic insuficiency
Malnutrition is directly related to a worse prognosis of CF patients. Good nutrition is important since it reduces the occurrence of respiratory infections and improves the quality of life. Moreover, in CF patients, energy needs are increased due to respiratory disease while calorie intake is decreased as a result of malabsorption and maldigestion. Pancreatic insufficiency is considered a fundamental factor that needs to be addressed. The delivery of active digestive enzymes to the proximal small intestine is a cornerstone of pancreatic exocrine insufficiency treatment in CF patients [36]. Many different dosing regimens were published, differing substantially in recommended doses of lipase. According to some, treatment with 500 U of lipase/kg/meal seems to result in good fat absorption while tolerated well [36]. Others recommend significantly higher doses – 10,000 unit/kg/day [8]. Some recommend administering enzymes to achieve a dose of up to 4,000 lipase units/g of ingested fat [37]. In general, the adequacy of pancreatic enzyme replacement therapy should be determined clinically, by monitoring nutritional status, signs and symptoms of malabsorption and excessive appetite with poor weight gain. Excessive doses of pancreatic enzymes may result in abdominal pain and constipation [9].
Acid supression plays more of a role in CF patients since bicarbonate secretion (by the pancreas, duodenum and biliary tree) is usually more disrupted than in other forms of CP [38,39]. Bicarbonate secretion causes an increase in pH of the duodenal content and thus allows the proper effect of the pancreatic enzymes because lipase is sensitive to low pH and release of pancreatic enzymes from the supplementation formulas is pH-dependent. However, CF patients seem to be particularly vulnerable to proton pump inhibitors (PPI) adverse effects and exposure to PPI therapy are associated with a higher number of hospitalisations for pulmonary exacerbation in CF patients [40]. Therefore, PPI should be used with caution and only in patients who do not respond to high doses of pancreatic enzymes and/or present with acid-related problems such as gastric/duodenal ulcers.
Even if all the guidelines of pancreatic supplementation are followed, fat absorption may not normalise. This may be caused by a decrease in fatty acid uptake by the abnormal intestinal mucosa (also caused by CF) [41]. Vitamin deficiencies may be present and CF patients should be monitored and supplemented accordingly [42].
Several novel therapeutic agents have recently been developed to increase CFTR protein activity and therefore, to specifically target the gene defect and not only to treat the resulting symptoms. The mode of action (thus also indications for their use) of individual molecules depends on the presence of a specific CFTR mutation. Ivacaftor acts as a CFTR protein potentiator – it increases the probability of CFTR channel opening and hence it facilitates chloride transport [43,44]. Lumacaftor acts as a CFTR protein corrector – it assists its formation and increases the amount of functional CFTR protein on the cell surface [45]. Tezacaftor is the next molecule currently available to correct CFTR protein structure [46]. These therapeutic agents are being tested and also used as combinations [45,46].
Submitted/Doručeno: 11. 6. 2019
Accepted/Přijato: 27. 7. 2019
doc. MUDr. Jan Trna, PhD.
Department of Gastroenterology
and Digestive Endoscopy,
Masaryk Memorial Cancer Institute Brno
Žlutý kopec 543/7
602 00 Brno, Czech Republic
jan.trna@seznam.cz
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Farrell PM, White TB, Ren CL et al. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J Pediatr 2017; 181: S4–S15. doi: 10.1016/j.jpeds.2016.09.064.
2. David J, Chrastina P, Pešková K et al. Epidemiology of rare diseases detected by newborn screening in the Czech Republic. Cent Eur J Public Health 2019; 27 (2): 153–159. doi: 10.21101/cejph.a5441.
3. Český registr cystické fibrózy. [online]. Dostupné na: www.cfregistr.cz.
4. Kopelman H, Corey M, Gaskin K et al. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology 1988; 95 (2): 349–355. doi: 10.1016/0016-5085 (88) 90490-8.
5. Whitcomb DC, Ermentrout GB. A mathematical model of the pancreatic duct cell generating high bicarbonate concentrations in pancreatic juice. Pancreas 2004; 29 (2): e30–e40.
6. Feigelson J, Pécau Y, Poquet M et al. Imaging changes in the pancreas in cystic fibrosis: a retrospective evaluation of 55 cases seen over a period of 9 years. J Pediatr Gastroenterol Nutr 2000; 30 (2): 145–151.
7. Votava F, Kožich V, Chrastina P et al. Výsledky rozšířeného novorozeneckého screeningu v České republice. Čes-slov Pediat 2014; 69 (2): 77–86.
8. Skalická V. Terapeutické trendy cystické fibrózy. Pediatr praxi 2014; 15 (6): 340–334.
9. Castellani C, Duff AJ, Bell SC et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros 2018; 17 (2): 153–178. doi: 10.1016/j.jcf.2018.02.006.
10. Taylor CJ, Chen K, Horvath K et al. ESPGHAN and NASPGHAN report on the assessment of exocrine pancreatic function and pancreatitis in children. J Pediatr Gastroenterol Nutr 2015; 61 (1): 144–153. doi: 10.1097/MPG.0000000000000830.
11. Elborn JS. Cystic fibrosis. Lancet 2016; 388 (10059): 2519–2531. doi: 10.1016/S0140-6736 (16) 00576-6.
12. Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am 2000; 84 (3): 597–607.
13. Uc A, Fishman DS. Pancreatic disorders. Pediatr Clin North Am 2017; 64 (3): 685–706. doi: 10.1016/j.pcl.2017.01.010.
14. Bonadia LC, de Lima Marson FA, Ribeiro JD et al. CFTR genotype and clinical outcomes of adult patients carried as cystic fibrosis disease. Gene 2014; 540 (2): 183–190. doi: 10.1016/ j.gene.2014.02.040.
15. Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993; 73 (7): 1251–1254. doi: 10.1016/0092-8674 (93) 90353-r.
16. Haardt M, Benharouga M, Lechardeur D et al. C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem 1999; 274 (31): 21873–21877. doi: 10.1074/jbc.274.31.21873.
17. Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet 2003; 67 (Pt 5): 471–485.
18. Stern RC. The diagnosis of cystic fibrosis. N Engl J Med 1997; 336 (7): 487–491. doi: 10.1056/NEJM199702133360707.
19. Fanen P, Wohlhuter-Haddad A, Hinzpeter A. Genetics of cystic fibrosis: CFTR mutation classifications toward genotype-based CF therapies. Int J Biochem Cell Biol 2014; 52: 94–102. doi: 10.1016/j.biocel.2014.02.023.
20. Durno C, Corey M, Zielenski J et al. Genotype and phenotype correlations in patients with cystic fibrosis and pancreatitis. Gastroenterology 2002; 123 (6): 1857–1864. doi: 10.1053/gast.2002.37042.
21. Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut 2007; 56 (8): 1153–1163. doi: 10.1136/gut.2004.062786.
22. Abu-El-Haija M, Valencia CA, Hornung L et al. Genetic variants in acute, acute recurrent and chronic pancreatitis affect the progression of disease in children. Pancreatology 2019; 19 (4): 535–540. doi: 10.1016/j.pan.2019.05.001.
23. Whitcomb DC. Value of genetic testing in the management of pancreatitis. Gut 2004; 53 (11): 1710–1717. doi: 10.1136/gut.2003.015511.
24. Cavestro GM, Zuppardo RA, Bertolini S et al. Connections between genetics and clinical data: role of MCP-1, CFTR, and SPINK-1 in the setting of acute, acute recurrent, and chronic pancreatitis. Am J Gastroenterol 2010; 105 (1): 199–206. doi: 10.1038/ajg.2009.611.
25. Vue PM, McFann K, Narkewicz MR. Genetic mutations in pediatric pancreatitis. Pancreas 2016; 45 (7): 992–996. doi: 10.1097/MPA.0000000000000589.
26. Bertin C, Pelletier AL, Vullierme MP et al. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. Am J Gastroenterol 2012; 107 (2): 311–317. doi: 10.1038/ajg.2011.424.
27. Ellis I. Genetic counseling for hereditary pancreatitis – the role of molecular genetics testing for the cationic trypsinogen gene, cystic fibrosis and serine protease inhibitor Kazal type 1. Gastroenterol Clin North Am 2004; 33 (4): 839–854. doi: 10.1016/j.gtc.2004.07.010.
28. Garg PK, Khajuria R, Kabra M et al. Association of SPINK1 gene mutation and CFTR gene polymorphisms in patients with pancreas divisum presenting with idiopathic pancreatitis. J Clin Gastroenterol 2009; 43 (9): 848–852. doi: 10.1097/MCG.0b013e3181a4e772.
29. Singh VK, Schwarzenberg SJ. Pancreatic insufficiency in cystic fibrosis. J Cyst Fibros 2017; 16 (Suppl 2): S70–S78. doi: 10.1016/j.jcf.2017.06.011.
30. De Boeck K, Weren M, Proesmans M et al. Pancreatitis among patients with cystic fibrosis: correlation with pancreatic status and genotype. Pediatrics 2005; 115 (4): e463–e469. doi: 10.1542/peds.2004-1764.
31. Choi JY, Muallem D, Kiselyov K et al. Aberrant CFTR-dependent HCO3-transport in mutations associated with cystic fibrosis. Nature 2001; 410 (6824): 94–97. doi: 10.1038/35065099.
32. Wilschanski M, Novak I. The cystic fibrosis of exocrine pancreas. Cold Spring Harb Perspect Med 2013; 3 (5): a009746. doi: 10.1101/cshperspect.a009746.
33.Whitcomb DC. Genetic polymorphisms in alcoholic pancreatitis. Dig Dis 2005; 23 (3–4): 247–254. doi: 10.1159/000090172.
34. Şişman G, Tuğcu M, Ayla K et al. Mutation analysis of PRSS1, SPINK1 and CFTR gene in patients with alcoholic and idiopathic chronic pancreatitis: a single center study. Turk J Gastroenterol 2015; 26 (2): 176–180. doi: 10.5152/tjg.2015.4287.
35. Midha S, Khajuria R, Shastri S et al. Idiopathic chronic pancreatitis in India: phenotypic characterisation and strong genetic susceptibility due to SPINK1 and CFTR gene mutations. Gut 2010; 59 (6): 800–807. doi: 10.1136/gut.2009.191239.
36. Van de Vijver E, Desager K, Mulberg AE et al. Treatment of infants and toddlers with cystic fibrosis-related pancreatic insufficiency and fat malabsorption with pancrelipase MT. J Pediatr Gastroenterol Nutr 2011; 53 (1): 61–64. doi: 10.1097/MPG.0b013e31820e208e.
37. Trapnell BC, Maguiness K, Graff GR et al. Efficacy and safety of Creon 24,000 in subjects with exocrine pancreatic insufficiency due to cystic fibrosis. J Cyst Fibros 2009; 8 (6): 370–377. doi: 10.1016/j.jcf.2009.08.008.
38. DiMagno EP. Gastric acid suppression and treatment of severe exocrine pancreatic insufficiency. Best Pract Res Clin Gastroenterol 2001; 15 (3): 477–486. doi: 10.1053/bega.2001.0195.
39. Gan KH, Geus WP, Bakker W et al. In vitro dissolution profiles of enteric-coated microsphere/microtablet pancreatin preparations at different pH values. Aliment Pharmacol Ther 1996; 10 (5): 771–775.
40. Ayoub F, Lascano J, Morelli G. Proton Pump inhibitor use is associated with an increased frequency of hospitalization in patients with cystic fibrosis. Gastroenterology Res 2017; 10 (5): 288–293. doi: 10.14740/gr917w.
41. Borowitz D, Durie PR, Clarke LL et al. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr 2005; 41 (3): 273–285.
42. Maqbool A, Stallings VA. Update on fat-soluble vitamins in cystic fibrosis. Curr Opin Pulm Med 2008; 14 (6): 574–581. doi: 10.1097/MCP.0b013e3283136787.
43. Condren ME, Bradshaw MD. Ivacaftor: a novel gene-based therapeutic approach for cystic fibrosis. J Pediatr Pharmacol Ther 2013; 18 (1): 8–13. doi: 10.5863/1551-6776-18.1.8.
44. Dřevínek P. Kauzální terapie cystické fibrózy. Postgrad med 2014; 16 (1): 21–22.
45. Brewington JJ, McPhail GL, Clancy JP. Lumacaftor alone and combined with ivacaftor: preclinical and clinical trial experience of F508del CFTR correction. Expert Rev Respir Med 2016; 10 (1): 5–17. doi: 10.1586/17476348.2016.1122527.
46. Walker S, Flume P, McNamara J et al. A phase 3 study of tezacaftor in combination with ivacaftor in children aged 6 through 11 years with cystic fibrosis. J Cyst Fibros 2019. In press. doi: 10.1016/j.jcf.2019.06.009.
47. Cuppens H. What is clinically relevant about the genetics of cystic fibrosis? In: Dominguez-Munoz JE (ed). Clinical pancreatology for practising gastroenterologists and surgeons. Blackwell Publishing 2005: 214–219.