Crohn's disease with interstitial pneumonia and seronegative spondyloarthropathy - a literature review and a case report
Dorota Ksiądzyna, Jan Cader, Leszek Paradowski Orcid.org 1
+ Pracoviště
SUMMARY
Ksiadzyna D, Cader J, Leszek Paradowski L. Crohn's disease with interstitial pneumonia and seronegative spondyloarthropathy - a literature review and a case report
Inflammatory bowel disease may coincide with extraintestinal manifestations including dermatologic, hepatobiliary, ophthalmic, nephrologic, musculoskeletal, pulmonary and thromboembolic disturbances. The authors report a case of Crohn's disease in a 45-year-old man with musculoskeletal and pulmonary disorders and a literature review concerning these extraintestinal manifestations in inflammatory bowel disease.
Key words: Crohn's disease - interstitial pneumonia - spondyloarthropathy.
SOUHRN
Ksi±dzyna D, Cader J, Leszek Paradowski L. Crohnova choroba provázená intersticiální pneumonií a seronegativní sponlyartropathií - literární přehled a popis případu
Nespecifické střevní záněty mohou být provázeny extraintestinálními projevy jako jsou kožní, hepatobiliární, oční, ledvinné, muskuloskeletální, plicní a tromboembolické komplikace. Autoři předkládají případ Crohnovy choroby u 45 letého muže s muskulosteletálními a plicními komplikacemi spolu s literárním přehledem extraintestinálních komplikací zánětlivých střevních chorob.
Klíčová slova: Crohnova choroba - intersticiální pneumonie - spondyloartropatie.
INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are chronic inflammatory diseases of the gastrointestinal tract. Although inflammatory bowel disease (IBD) primarily involves the bowel, UC and CD are associated with extraintestinal manifestations, which for some patients may be even more troublesome than the bowel disease itself. The extraintestinal manifestations can be divided into those, in which the clinical activity follows the activity of the bowel disease and others unrelated to the clinical activity of the bowel disease(1). Most extraintestinal manifestations occur more commonly with Crohn's colitis than Crohn's disease confined to the small intestine(2). Although musculoskeletal disorders appear relatively frequently, diseases of the respiratory system are reported less often and coexistence of these both extraintestinal manifestations seems to be rare as it is presented in a literature review following a case report.
CASE REPORT
A 45-year-old man with unremarkable family history was admitted to the Department of Gastroenterology and Hepatology of the Medical University in Wrocław, Poland in January 2006 to establish a final diagnosis of chronic colitis he has been suffering from for 5 years. He was healthy until approximately 7 years ago, when he first began to experience inguinal and low back pain with no relation to rest. After a diagnosis of thoracic and lumbar spondylosis with lumbalgia was made in June 1999, he was treated with physiotherapy and nonsteroidal anti-inflammatory drugs (NSAID). Eventually, because of a lack of long-term improvement he was referred to a rheumatologist in November 2000, who found sigmoid thoracic scoliosis, signs of left-side sacroiliitis and impaired flexibility of thoracic part of the spinal column. Blood examination disclosed no rheumatoid factor, negative Waaler-Rose and latex reaction, elevated erythrocyte sedimentation rate (ESR) of 56 mm/h and C-reactive protein (24 mg/l). X-ray examination revealed blurred structure of the left sacroiliac articular space, spondylodiscitis within a few levels of the thoracic part of the spinal column, squaring of the vertebral bodies and syndesmophytes. The tentative diagnosis of seronegative spondyloarthropathy with a picture consistent with ankylosing spondylitis was established. The patient has been started on sulfasalazine (2times 500 mg-3times 500 mg-2times 1000 mg daily), folic acid (15 mg daily), NSAID and intensive physiotherapy. The first follow-up examination after 3 weeks proved good compliance and drug tolerance but still painful left-side ilium, stifft left-side sacroiliac joint and high ESR (49 mm/h). During the next follow-up visit the patient reported quite good general feeling, decreased spinal stiffness, no morning stiffness, recurrent left hip pain and weight loss (2 kg). Apart from markedly elevated ESR (30 mm/h), lymphocytosis in a blood smear (56 %, total WBC count 7,4 g/l) and hypercholesterolaemia (292 mg/dl) blood tests were normal. In June 2001 right-side coxitis was diagnosed. The patient also developed exudative right-side gonitis. He occasionally required corticosteroid therapy to control symptoms (prednisone orally 25 mg daily, methyloprednisolone 80 mg intra-articularly). When chronic diarrhoea occured the rheumatologist suggested enteroarthropathy.
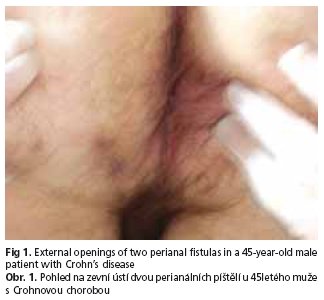
In May 2002 the patient was admitted to the Surgery Department of a municipal hospital with chief complaints of generalized malaise, fatigue, chronic diarrhoea of approximately twelve months' duration, occasional anal bleeding, bloody stool and dyschezia. Significant laboratory findings showed elevated ESR (50 mm/h) and mild anaemia (Hb 12.8 g/dl). Endoscopic examination of the colon revealed, hyperaemic mucosa with erosions extending from the transverse colon to the anus, anal fissure and haemorrhoids grade II/III. Histologic examination showed chronic active colitis, suggesting UC, if consistent with the clinical picture and laboratory findings. Such a diagnosis was established and sulfasalazine treatment was ordered. The patient underwent three follow-up examinations (October 2002, June 2003, June 2004) which proved good compliance and drug tolerance. Laboratory findings included mild anaemia (Hb ranging from 12.1 to 12.6 g/dl, Hct 36 %) and a decrease of ESR to 28 mm/h. Colonoscopy revealed mild mucosal hyperaemia without erosions, good susceptibility to insufflation and normal haustration. In 2003 patchy hyperaemic changes and softening of the mucosa were confined only to rectum. Thickening at the level of pectiniform line was found and excised. Its histologic examination showed anal mucosa with granulomatous inflammation. The dignosis of UC was sustained, mesalazine topically and sulfasalazine orally were applied. One year later segmental changes mostly in the sigmoid colon and rectum in the form of hyperaemia with very well visible vascular pattern were observed. In March 2005 the patient was admitted again with complaints of local painful thickening in the left anal region with mild anaemia (Hb 12.6 g/dl, Hct 36.7 %) and a total WBC count of 10.3 g/l in laboratory tests. Colonoscopy revealed hyperaemic mucosa of the caecum with numerous erosiones, sharply outlined vascular pattern in the descending colon and internal opening of the fistula on the lateral wall of the rectum (fig 1). The suspicion of CD emerged. Antibiotics (amoxicillin + clavulanic acid, azithromycin) were administered orally. In December 2005 he also underwent ciprofloxacin (2times 500 mg orally) and metronidazole (2times 500 mg orally) course, which ameliorated his general feeling due to the reduction of low abdominal pain, flatulence and purulent discharge from the perianal fistula.
Meanwhile, in February 2004 he was admitted to Alergology and Internal Medicine Department of a municipal hospital to diagnose asymptomatic pulmonary changes found incidentally on X-ray examination. Significant laboratory findings included only high ESR (44 mm/h), mild anaemia (Hb 12.5 g/dl) and oxygen saturation of 93.6 %. Spirometry was normal. High resolution computed tomography (HRCT) of the chest showed a cluster of fasciculoreticular and micronodular condensed densities partially connecting with pleura and localized peripherally in an apical second segment of the right lung. Mediastinal lymphonodes were not enlarged and both suprarenal glands were normal. The character of changes observed was in favour of postinflammatory changes, however because of the risk of proliferative neoplastic changes a follow-up examination was ordered. Control HRCT in January 2005 revealed almost complete resorption of the lesions with only residual single fascicular and micronodular cicatrical changes, but in a third segment of the right lung small irregular region of a consolidation of parenchymal substance and parenchymal infiltrations, sometimes in the form of nodular changes appeared. In the inferior part of this region fine aerial bronchogram was observed. It was assessed as the picture of infiltrative inflammatory-atelectatic changes, during the course of a specific inflammation. The patient was referred to the Clinical Department of Pulmonology and Lung Tumors in February 2005 where he underwent blood tests (Hb 12.3 g/dl, Hct 37.7 %, fibrinogen 6.58 g/l, normal blood gas analysis), tuberculin reaction (within a normal range), oxygen saturation (90.6 %), chest X-ray examination (in the upper lung field on the border with the middle field peripherally in the right lung patchy opacity), bronchofiberoscopy, spirometry, examination of the sputum (BK-negative), bronchial secretion, bronchial washings and brush biopsy. Tuberculosis and neoplastic tumor of the lung were excluded. In follow-up chest CT examination in May 2005 fine fasciculo-micronodular densities, probably postinflammatory changes were present. In addition, a parenchymal consolidation with the thickening of the transverse fissure of the right lung and aerial bronchogram peripherally in a base of a third segment of the right lung were revealed. It was assessed as progression in comparison with the previous CT. Anyway, the leasions were still not characteristic, suggestive of an inflammation rather than a specific infection or a neoplastic process. The transbronchial biopsy of the upper lobe of the right lung was performed and its histologic examination disclosed not very abundant interstitial inflammatory infiltration composed mostly of lymphocytes and fine granulomas with polynuclear giant cells. In laboratory findings anaemia (Hb 11.5 g/dl, Hct 35.1 %) and hyperfibrinogenaemia (6.4 g/l) were still observed. There were also BK-negative sputum, no evidence of a neoplasm in a cytologic examination, normal oxygen saturation and gas diffusion and only Streptococcus orale, Neisseria spp. growth in a bacteriological culture. The final diagnosis of interstitial pneumonia as an extraintestinal manifestation of IBD was established and the patient was started on 40 mg prednisone daily. The follow-up examination after 6 weeks showed good steroid tolerance, general improvement, regression of joint pain. The serum level of fibrinogen decreased (5.0 g/l), but pulmonary changes were still visible on chest X-ray. However, next two follow-up examinations after 6 and 12 weeks showed partial but evident regression of pulmonary changes on X-ray films, which enabled to reduce prednisone's dose gradually to 15 mg daily in October 2005. However, in December 2005 a higher dose of prednisone (20 mg daily) was introduced because of the recurrence of patchy opacity in the upper right lung field despite of complete regression of opacities in the location of the small interlobal fissure.
On admission to our department the patient complained of dysgeusia, occasional low back pain, coxalgia and omalgia. He described the activity of the perianal fistula as very low compared with the time when it was identified for the first time. The patient denied any other symptoms, but he reported a weight gain of about 10 kg over the past 7 months which was from the time prednisone was started on a regular basis. His bowel movements were regular (twice daily) with no patologic constituents; no abdominal pain, no fever. He had been smoking 30 cigarettes daily during 10 years, but he quited 8 years ago. He denied alcohol abuse whenever in his life. On physical examination the patient was in a good emotional and somatic condition. His BMI was 28.7 kg/m2, HR = 72/min, RR= 110/80 mm Hg. The physical examination was remarkable only for the presence of external openings of two perianal fistulas with a very small purulent discharge on compression and a slight thickening of the skin in this region. Significant laboratory findings included only high level of total cholesterol (304 mg/dl), cholesterol LDL (188 mg/dl), triglyceride (184 mg/dl) and fibrinogen (4.4 g/l). HLA B27 was negative. Urinalysis and stool examination were normal. Bacteriological culture of the stool for the presence of Campylobacter spp., Yersinia spp. as well as Salmonella spp., Shigella spp. was negative. The culture was positive for Escherichia coli, Enterococcus faecalis, Proteus mirabilis and Candida albicans (scant growth). Discharge from the external orifice of the perianal fistulas was negative for anaerobic bacteria, positive for Escherichia coli, Enterococcus fecalis and Proteus mirabilis. On abdominal ultrasonography slight hepatomegaly, an increase of hepatic echogenicity with no focal changes suggesting steatosis, and duplication of pyelocalyceal complex of the left kidney were shown. Esophagogastroduodenoscopy revealed gastritis and colonoscopy - hyperaemia of the ileocaecal valve, slight blurring of the vascular patern and single aphthoid erosions in the caecum and ascending colon. Normal view of the small bowel evaluated by enteroclysis was obtained. X-ray examination of the spinal column and sacroiliac joints disclosed osteoporosis, spondylosis deformans and bilateral partial blurring of the sacroiliac articular space. Densitometry of the neck of the left femoral bone revealed osteopenia [T-score (-) 1.02]. On the basis of patient's history, clinical picture, laboratory findings and examinations the final diagnosis of fistulising Crohn's colitis in remission (CDAI = 89) with extraintestinal manifestations such as seronegative spondyloarthropathy and interstitial pneumonia was established. Following surgical consultation the patient was qualified for a conservative treatment with corticosteroids, sulfasalazine, folic acid, potassium, calcium, vitamin D3, NSAID when necessary, proton pump inhibitor, balanced, low-fat diet, dressing change locally in the left perianal region when required and physiotherapy. The patient was discharged from the hospital in a good general state with an advice to check up regularly in the gastroenterological, pulmonary and rheumatological outpatient clinics.
DISCUSSION
Apart from anaemia, subfebrile body temperature and weight loss extraintestinal manifestations of IBD comprise musculoskeletal system, skin, liver, biliary tract and pancreas, less frequently kidney, heart, lungs, organ of vision and central nervous system. Musculoskeletal disorders belong to the most common extraintestinal manifestations in IBD(3). Wide ranges of prevalence have been reported depending on the criteria used to define specific clinical entities and on the selection of patients. According to European Collaborative IBD Study Group 53 (33.1 %) of 160 IBD patients experienced at least one musculoskeletal manifestation. In an inceptional cohort of newly diagnosed IBD patients, musculoskeletal manifestations were observed in 30.7 % of the patients(4). They may be divided into peripheral arthritis (migratory polyarthritis, monoarthritis, arthralgia) and axial arthritis and ankylosing spondylitis. The spectrum of muscoloskeletal manifestations in IBD patients also includes all of the clinical features of inflammatory spinal pain, dactylitis, enthesitis (Achilles tendinitis and plantar fasciitis), buttock pain and anterior chest wall pain. Other clinical entities encompass clubbing, osteomalacia, osteopenia and osteoporosis.
The articular manifestations begin either concomitantly or subsequent to the bowel disease; however, the onset of spinal disease often precedes the diagnosis of IBD. The prevalence of different muscoloskeletal manifestations is similar in UC and CD.
Monoarticular arthritis encompasses usually a knee or an ankle joint, but sometimes also hips, elbows and wrists, whereas migratory polyarthritis tends to affect at least two but usually fewer than six joints at the same time. It is more common with Crohn's colitis than UC and is uncommon with Crohn's disease confined to the small intestine that is the case pertaining to the patient described.
Arthritis rarely precedes the intestinal manifestation of the disease or complicates the quiescent bowel disease. Most flares of the arthritis last only a few weeks leading to deformity with radiologic changes in fewer than 25 % of cases. Successful treatment of the intestinal inflammation results in improvement of the arthritis. Colitic arthritis responds well to corticosteroids and the treatment of bowel disease with corticosteroids often results in a dramatic improvement in the associated arthritis. None of them cause permanent joint damage and each subsides as the activity of the bowel disease remits. Such complications as perianal disease, pseudopolyposis, pyoderma gangrenosum seem to be associated with an increased frequency of arthritis.
Sacroiliitis is an inflammation of the joint between the sacrum and the ilium. It can be seen either in conjunction with ankylosing spondylitis or, more often, as an isolated disorder. Most patients are asymptomatic in contrast to ankylosing spondylitis, but some complain of low back pain, which may be progressing slowly with no relation to the activity of intestinal inflammation. In many cases the diagnosis is made incidentally by radiography that reveals blurring of the margins of the sacroiliac joints with pathy sclerosis. In Turkcapar et al study 13.6 % of patients were asymptomatic for spondyloarthropathy and their sacroiliac radiograms and computed tomography (CT) showed grade 2 sacroiliitis(5). Sometimes sacroiliitis may progress to ankylosing spondylitis and its symptoms procede IBD. However, the majority of patients are negative for HLA B27 and do not progress to ankylosing spondylitis which occurs in 1-2 % of mostly male patients with IBD.
The activity of ankylosing spondylitis does not follow that of the bowel disease, and treatment of the bowel disease does not effect the spondylitis. Ankylosing spondylitis presents with morning stiffness, low back pain and stooped posture. Radiography of the spine shows squaring of the vertebrae and strightening of the spine. Syndesmophytes appear along the lateral and anterior portions of the intervertebral discs, giving the picture of »bamboo spine«. Patients with UC have a 30-fold increase in the incidence of ankylosing spondylitis compared with the general population. Even though there is no increased incidence of HLA-B27 in UC, 80 % of those with both UC and ankylosisng spondylitis are HLA-B27 positive. By definition, the latex test is negative. In contrast to colitic arthritis, which is episodic and usually nondeforming, ankylosing spondylitis can be relentlessly progressive and crippling. The results of medical management of the disease are usually poor. NSAID reduce inflammation and pain, but do not halt the progression of the disease. Physiotherapy plays a major role in maintaining function. Regular range of motion excercises prevent the development of contractures and preserve joint function. A physical and occupational therapist can teach patients to bend or to carry objects in a way that is least likely to damage inflammed joints.
Clubbing seen primarily in extensive small bowel involvement is other musculoskeletal manifestation of CD(6). It may be related to bowel inflammation activity and may even regress in some cases after surgical treatment. In addition, CD patients are at risk of pelvic osteomyelitis when fistulazing small bowel disease extends into the pelvic brim and hip joint.
The etiopathogenesis of muscoloskeletal manifestations in IBD is unclear. Several arguments favour an important role of the intestinal mucosa in the development of spondylarthropathy. The natural history is characterized by periods of flares and remissions; therefore, the efficacy of treatment is difficult to establish. Most patients respond to rest, physical therapy and NSAID, but these drugs may activate bowel disease. At the moment sulfasalazine and mesalazine are the first choice treatment. Several immunosuppressive and biological agents including methotrexate, thalidomide and TNF-alpha antagonists have efficacy for both articular and intestinal inflammation and are currently in use for the induction of remission and for maintenance in more severe cases(7).
Other musculoskeletal manifestations like osteomalacia, osteopenia or osteoporosis are related to metabolic rearrangements or therapeutic side-effects (mostly corticosteroids' use). Data on low bone mineral density (BMD) in IBD vary considerably from 31 to 59 %(8). Osteopenia or osteoporosis occur in some populations in as many as half of patients with CD(9). In the study of 54 CD and 49 UC patients reduced BMD values were found in 48 % of CD, and in 38 % of UC patients(10). Compared with control subjects, the mean BMD was significantly lower in CD and UC patients, positively correlated with BMI and inversely with the lifetime steroid dose. The evolution of BMD suggests that low bone density is associated with the pathogenesis of CD, whereas in UC it seems to be correlated with the side effects of corticosteroid treatment. Even in patients with minimal corticosteroids' exposure cortical and trabecular bone loss may approach 1-2.5 % of bone mass per year(11). Like in the case presented, in CD osteopenia may be present at diagnosis. Numerous factors may contribute including malnutrition, malabsorption, smoking, persistent inflammation (TNF and IL-1 enhance bone resorption) and steroids' use, especially in UC. Although osteopenia may occur independently of steroid use, steroids can still worsen the problem. Estrogen replacement therapy in post-menopausal women, raloxifene, calcium, vitamin D3, calcitonin, bisphosphonates - alendronate and risedronate are therapeutic options, which can considerably augment bone density(12). Guidelines for the screeining and management of osteoporosis in IBD have been developed by the British Sociaty of Gastrenterology(13).
Respiratory involvement in patients with IBD has been reported since 1976 (acc. to 14). Data on the incidence of pulmonary changes in IBD are inconsistent. In Herrlinger et al study 35 patients with CD, 31 with UC and 30 control patients were investigated with respect to the following pulmonary function tests: forced expiratory volume in 1 sec. (FEV1), inspiratory vital capacity (IVC), Tiffeneau value (FEV1/IVC), and lung CO transfer capacity - D(LCO) (15). 39 % of CD patients and 45 % of UC patients but only one of the controls exhibited at least one pathological (< 80 % of predicted value) pulmonary function test. The results were worse during an active phase of the disease than in remission. Some authors claim that pulmonary changes in IBD are relatively frequent, but are not diagnosed because of their subclinical course. Fireman et al found that among CD patients without respiratory symptoms there was a high incidence (65 %) of lung involvement(16). Heatley et al. disclosed restrictive and obturative changes on spirometry in 50 % of 102 patients with IBD(17). Marvisi et al. observed pulmonary functional disorders in 17 out of 32 asymptiomatic patients with UC with no changes in HRCT, usually in the form of disturbed carbon monoxide diffusing capacity suggesting the involvement of pulmonary interstitium(18). The grade of the pulmonary functional disturbances correlate with the bowel involvement assessed on histologic examination. Allergic symptoms, respiratory symptoms, abnormal lung function tests and skin prick test positivity were more common among the IBD patients in comparison with the controls(19). Pulmonary abnormalities in UC and CD can present years after the onset of the bowel disease and affect any part of the lungs. Early recognition is important as they can be strikingly steroid-responsive(20).
Patterns of involvement include:
- respiratory tract inflammation (subglottic stenosis, chronic bronchitis, severe chronic bronchial suppuration, bronchiectasis, chronic bronchiolitis);
- varied patterns of interstitial lung disease, mainly bronchiolitis obliterans with organizing pneumonia, and pulmonary infiltrates and eosinophilia;
- miscellaneous (neutrophilic necrotic parenchymal nodules, serositis) (14).
Still discussed the etiopathogenesis of pulmonary changes is often referred to the population of lymphocytes of the respiratory epithelium, which activity may be induced by intestinal changes. Lymphocytic infiltrations in the respiratory epithelium are present in 18 % of CD patients with no clinical and radiological signs of pulmonary involvement(21). Storch et al. suggest the role of free radicals produced by alveolar macrophages in the interstitial changes(22). It is also important to remember that some of the pulmonary lesions may be of iatrogenic orgin due to mesalazine, sulfasalazine, azathioprine and methotrexate use(23). They are usually reversible and susceptible to the steroid therapy. Unfortunately, none of the pulmonary changes such as obliterative bronchiolitis, interstitial pneumonia, focal pulmonary fibrosis, pleural exudate, abscess, caseation with functional consequences like decreased CO diffuse capacity or hypoxaemia are pathognomonic for IBD(24,25) what proves the need for careful differential diagnosis including specific and non-specific inflammation as well as neoplastic process of the lung as it has been done in case of our patient.
To conclude, the clinical picture of IBD may vary considerably as years pass so the final diagnosis concerning the type of IBD (UC or CD) may be established long time after its first symptoms. Extraintestinal manifestations of IBD do occur and change with the bowel disease course. Apart from relatively frequent disorders such as for example enteroarthropathy some rare entities like interstitial pneumonia may appear. Some of the extraintestinal manifestations may be clinically silent and thus difficult to diagnose. It is important to remember that patients with IBD can develop varied inflammatory complications and a sizable fraction of these complications is steroid-sensitive. In addition, IBD treatment is not indifferent to the patient's general condition and may even pose a risk of iatrogenic disorders like bone mineral loss due to steroidotherapy.
References
1. Bartnik W. Jelito grube. In: Konturek SJ, ed. Gastroenterologia i hepatologia kliniczna. Warszawa: PZWL 2001: 385-407.
2. Stenson WF. Inflammatory bowel disease. In: Yamada T, Alpers DH, Laine L, et al. (eds). Textbook of Gastroenterology. Volume 2. Philadelphia: Lippincott Williams & Wilkins 1999: 1775-1839.
3. Salvarani C, Vlachonikolis IG, van der Heijde DM, et al. Musculoskeletal manifestations in a population-based cohort of inflammatory bowel disease patients. Scand J Gastroenterol 2001; 36(12): 1307-1313.
4. Salvarani C, Fornaciari G, Beltrami M, et al. Musculoskeletal manifestations in inflammatory bowel disease. Eur J Intern Med 2000; 11(4): 210-214.
5. Turkcapar N, Toruner M, Soycan I, et al. The prevalence of the extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol Int 2005; 1: 1-6.
6. Farmer RG, Hawk WA, Turnball RB. Clinical patterns in Crohn's disease: a statistical study of 615 cases. Gastroenterology 1975; 68: 627-635.
7. Generini S, Fiori G, Matucci-Cerinic M. Therapy of spondyloarthropathy in inflammatory bowel disease. Clin Exp Rheumatol 2002; 20(6;Suppl 28): S88-94.
8. Valentine JF, Sninsky CA. Prevention and treatment of osteoporosis in patients with inflammatory bowel disease. Am J Gastroenterol 1999; 94: 878-883.
9. Schoon EJ, van Nunen AB, Wouters RS, et al. Osteopenia and osteoporosis in Crohn's disease: prevalence in Duch population-based study cohort. Scand J Gastroenterol 2000; 232(Suppl): 43-47.
10. Dinca M, Fries W, LuisettoG, et al. Evolution of osteopenia in inflammatory bowel disease. Am J Gastroenterol 1999; 94(5): 1292-1297.
11. Giacomin D, Fries W, Luisetto G, et al. Bone alterations in Crohn's disease: a comparison between active and quiescent disease (abstract). Gastroenterology 1994; 107: A686.
12. Haderslev KV, Tiellesen L, Tjellesen HA, et al. Alendronate increases lumbar spine bone mineral density in patients with Crohn's disease. Gastroenterology 2000; 119: 639-646.
13. Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Gastroenterology Society. Eur J Gastroenterol Hepatol 1998; 10(8): 689-696.
14. Camus P, Piard F, Ashcroft T, et al. The lung in inflammatory bowel disease. Medicine. 1993; 72 (3): 151-83.
15. Herrlinger KR, Noftz MK, Dalhoff K, et al. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol 2002; 97: 377-381.
16. Fireman Z, Osipov A, Kivity S, et al. The use of induced sputum in the assessement of pulmonary involvement in Crohn's disease. Am J Gastroenterol 2000; 95: 730-734.
17. Heatley RV, Thomas P, Prokipchuk EJ, et al. Pulmonary function abnormalities in patients with inflammatory bowel disease. QJM 1982; 51: 241-250.
18. Marvisi M, Borrello PD, Brianti M, et al. Changes in the carbon monoxide diffusing capacity of the lung in ulcerative colitis. Eur Respir J 2000; 16: 965-968.
19. Ceyhan BB, Karakurt S, Cervik H, et al. Bronchial hyperreactivity and allergic status in inflammatory bowel disease. Respiration 2003; 70(1): 60-66.
20. Mahaceva R, Walsh G, Flower CD, et al. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur Respir J 2000; 15(1): 41-48.
21. Munck A, Murciano D, Pariente R, et al. Latent pulmonary function abnormalities in children with Crohn's disease. Eur Respir J 1995; 8: 377-380.
22. Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel diseases. Inflamm. Bowel Dis 2003; 9: 104-115.
23. Haralambou G, Teirstein AS, Gil J, et al. Bronchiolitis obliterans in a patient with ulcerative colitis receiving mesalazine. Mt Sinai J Med 2001; 68: 384-388.
24. Eade OE, Smith CL, Alexander JR, et al. Pulmonary function in patients with inflammatory bowel disease. Am J Gastroenterol 1980; 3: 154-156.
25. Kuzela L, Vavrecka A, Prikazska M, et al. Pulmonary complications in patients with inflammatory bowel disease. Hepatogastroenterology 1999; 46: 1714-1719.
Pro přístup k článku se, prosím, registrujte.
Výhody pro předplatitele
Výhody pro přihlášené