Urolithiasis in Patients with Inflammatory Bowel Diseases
Vladimír Teplan Orcid.org 1,2,3, Milan Lukáš Orcid.org 4
+ Affiliation
Summary
Inflammatory bowel diseases are typically accompanied by diarrhoea and malabsorption, both of which are predisposing factors for the formation of renal calculi. In patients who have not undergone bowel surgery the prevalence of urolithiasis has ranged from 1.5% to 5%, but after surgery stone prevalence can increase to up to 16%. Enteric hyperoxaluria is a frequent complication of inflammatory bowel diseases, ileal resection and Roux-En-Y gastric bypass and is a recognised cause of nephrolithiasis and nephrocalcinosis. The excess of oxalate is primarily excreted by the kidneys. Increased urinary excretion of oxalate results in urinary calcium oxalate supersaturation, leading to crystal aggregation, urolithiasis, and/or nephrocalcinosis. Prevention of oxalate lithiasis includes high fluid intake, prescription of oral citrate and magnesium, calcium supplement, nutritionally balanced low-oxalate low-fat diet and also biological manipulation of intestinal flora (Oxalobacter formigenes, Bifidobacterium lactis, etc.). New therapeutic approaches to patients with inflammatory bowel diseases have completely changed the natural history of these diseases. Whether this has changed the prevalence and risk factors for urinary calculi in patients with inflammatory bowel diseases is still unknown.
Keywords
hyperoxaluria, prevention, intestinal flora, urolithiasis, urolithiasis, inflammatory bowel diseasesIntroduction
Inflammatory bowel diseases (IBD) are chronic diseases comprising Crohn’s disease (CD) and ulcerative colitis (UC) resulting from a disregulated immune response in genetically susceptible individuals [1].
IBD are typically accompanied by diarrhoea and malabsorption, both of which are predisposing factors for the formation of renal calculi, e. g. a great- er than expected number of patients with asymptomatic and symptomatic urolithiasis were noticed in IBD clinical practice. Patients with IBD are thought to develop nephrolithiasis more often than the general population [2,3]. Among those patients who have not had bowel surgery the prevalence of urolithiasis has ranged from 1.5% to 5% [4–10], which is very similar to the usual values for stone prevalence rates in the United States (3% to 5%) [11]. However, given bowel surgery, rates are approximately two- to three-fold higher giving a stone prevalence of 3.7% to 16% for surgeries combined [4,7].
To date, few data are available about urolithiasis in IBD and previous studies have reported a wide variation in the incidence of urolithiasis. New therapeutic approaches to patients with IBD have emerged recently and these have completely changed the natural history of these diseases. These ap- proaches are aimed not only at lowering the rates of surgery but also at better prognoses, fewer hospitalisations, and improved quality of life [5–8]. Whether this has changed the prevalence and risk factors for urinary calculi in patients with CD is still unknown. Determining clinical risk factors for the development of urinary stones in patients with CD might uncover new strategies for treatment and prevention.
Malabsorption, and malabsorption secondary to bowel resection are factors associated with IBD that predispose these patients to urinary calculi formation [12–14]. As a result, they often present with multiple stones and are at risk of stone recurrence if the underlying causes are not adequately addressed.
UC is the most common form of IBD worldwide. Despite advances in medical therapy, approximately 30% of patients with UC eventually require colectomy [15,16]. Restorative proctocolectomy with ileal pouch-anal anastomosis has become a standard of care in UC patients following colectomy. While restorative proctocolectomy with ileal pouch-anal anastomosis improves a patient’s health-related quality of life, a number of metabolic complications can occur, including bone loss anaemia and vitamin D deficiency [17–19]. Patients with IBD are known to have a high frequency of nephrolithiasis. The reported frequency of nephrolithiasis ranges from 0.2% to 11.0% in non-colectomy UC patients and from 8.4% to 40.0% in UC patients with total colectomy and ileostomy [20–23]. Similarly, the report- ed frequency of nephrolithiasis ranges from 4.0% to 5.5% in patients with CD without bowel resection surgery and from 15.0% to 30.5% in CD patients after small bowel surgery [22,23].
Various fluid and electrolyte changes in these patients increase the risk of nephrolithiasis. Low urine volume, pH, and urine citrate and magnesium levels along with high urine oxalate are speculated to be the main mechanisms for urinary supersaturation and nephrolithiasis in IBD patients. It is anticipated that pouch patients (with a prior history of IBD) after undergoing bowel-anatomy-altering surgery may have a further increase in risk of nephrolithiasis.
Enteric hyperoxaluria in IBD
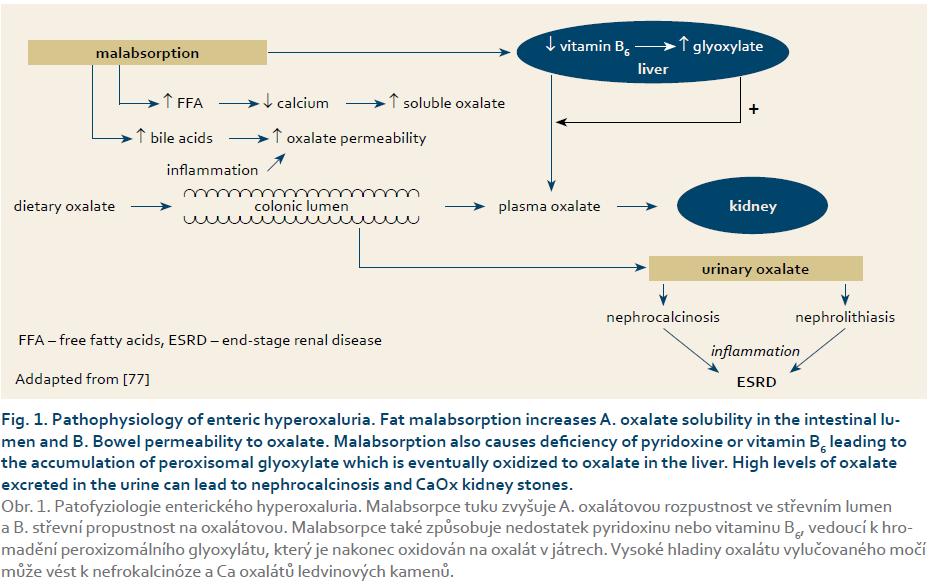
Hyperoxaluria is generally a rare metabolic disorder characterised by calcium oxalate deposition in different tissues. It is caused either by an inherited disease of oxalate metabolism (PH – primary hyperoxaluria) [24] or by an acquired disturbance (SH –secondary hyperoxaluria) [25]. Oxalate can bind with various cations, such as sodium, potassium, magnesium, and calcium. Even though sodium oxalate, potassium oxalate, and magnesium oxalate are water soluble, calcium oxalate (CaOx) is almost insoluble [26]. The excess oxalate is primarily excreted by the kidneys. Increased urinary excretion of oxalate results in urinary CaOx supersaturation, leading to crystal aggregation, urolithiasis, and/or nephrocalcinosis. CaOx crystals are typically deposited within the renal interstitium and renal tubule cells. When the glomerular filtration rate falls below 30–40 mL/min per 1.73 sqm, plasma oxalate levels increase due to reduced urinary oxalate excretion. Oxalate nephropathy should be considered in the differential diagnosis of acute renal failure, especially when previous renal impairment and fat malabsorption are present. Oxalosis represents the histological manifestation of crystalline deposits of CaOx in various tissues and organs. This phenomenon typically occurs when plasma oxalate exceeds 30 μmol/L, which represents its plasma supersaturation threshold, resulting in deposition into several tissues, including the retina, myocardium, vessel walls, skin, bone, and the central nervous system. Deposition of these crystals in the kidneys may cause either acute kidney injury, or may lead to the formation of diffuse renal calcifications (nephrocalcinosis) and stones (nephrolithiasis) in the long term. Patients presenting with renal calculi require screening for hyperoxaluria. In order to minimise renal damage, patients suffering from SH should be promptly identified and appropriately treated. Enteric hyperoxaluria is a frequent complication of IBD, ileal resection and RYGB and is a recognised cause of nephrolithiasis and nephrocalcinosis. Less well-known is that it also contributes to chronic kidney disease and end-stage kidney disease. The urinary solubility product of CaOx, a determinant of the tendency of urine to yield crystals, is 10× more affected by a rise of urinary oxalate concentration than an equimolar rise in urinary calcium concentration [27]. The prevalence of hyperoxaluria has been estimated at 5–24% of all patients with gastrointestinal diseases associated with malabsorption [28,29]. Hyperoxaluria is becoming more common, secondary to an increase in IBD [30] and bariatric surgery. The associated prevalence of chronic kidney disease and end-stage kidney disease is less clear but may be more consequential than recognised.
IBD and urinary tract infection
Infection is also a common complication of IBD, and compounding factors such as immunosuppression [31] and malnutrition [32,33] put these patients at risk for severe clinical sequelae. Not surprisingly, a recent population-based study found a four-fold increase in mortality among IBD patients requiring hospitalisation for infection [34]. Interestingly, urinary tract infections (UTIs) were the most frequently reported infection among these patients. IBD is also associated with unusual genito-urinary complications, such that both infection and urolithiasis are commonly seen in patients with IBD. Furthermore, IBD patients with infected calculi have much higher odds of developing significant clinical sequelae, including higher rates of end-organ failure, sepsis, and hospital admission. It was also found that IBD patients with upper tract urinary calculi had significantly higher odds of either acute cystitis or pyelonephritis than the general stone population.
Furthermore, an IBD diagnosis was an independent predictor for sepsis with almost two-fold higher odds of urosepsis than those of the non-IBD stone population. Given that previous literature reviewing infections in IBD patients has demonstrated that patients treated with infliximab, prednisone, and immunomodulators such as azathioprine and 6-mercaptopurine are at increased risk of opportunistic and serious infections [31,32,35], as well as evidence that bacterial translocation as well as structural abnormalities, such as enterovesical fistulas, may cause UTIs more frequently in IBD patients [36].
Another important finding is the high rate of renal failure among IBD patients. This finding is likely multifactorial in nature, including underlying intrinsic renal disease, acute obstruction, prerenal azotemia, and exposure to nephrotoxic therapies. Potential nephrologic and urologic complications of IBD outside of stone disease include noncalculus obstruction secondary to retroperitoneal fibrosis or ureteral stricture, parenchymal disease from nephrocalcinosis, distal renal tubular acidosis, and interstitial nephritis, as well as nephrotoxicity from certain medical treatments [37]. In a matched cohort study by Primas et al. [38], recurrent urolithiasis and the number of interventions due to kidney stones were significant risk factors for renal insufficiency in IBD patients. The previously mentioned genito-urinary and renal complications of IBD along with chronic dehydration likely render these patients less able to physiologically mitigate an acute kidney injury associated with obstruction and/or infection from urinary calculi. Unfortunately, urolithiasis is often asymptomatic and may go undetected until passage or obstruction occurs [39]. In the case of IBD patients, even when stone symptoms are present, they may be overshadowed by concurrent bowel symptoms, increasing the chance of missed detection. These patients may also be unaware that their IBD puts them at risk of nephrolithiasis [40]. In a prospective study of newly diagnosed IBD patients, Cury et al. [39] found 38% had asymptomatic stone formation and 10% had hydronephrosis on screening ultrasound. Given this high rate of nephrolithiasis in the general IBD population and the findings that there is significant morbidity associated with infected urolithiasis in this group, efforts at early identification are merited. These patients may form a select group who urologists should consider for elective stone treatment, even if asymptomatic. IBD had concomitant hydronephrosis and multiple urinary calculi population.
Gastroenterologists should consider urine microscopy or ultrasound to identify urolithiasis in their IBD patients. Similar screening guidelines for osteoporosis in IBD patients were published in 2003 and resulted in significant im- provements in the detection of osteopenia and osteoporosis in these patients, whereas the level of care given by gastroenterologists in treating these conditions has improved [41]. Screening for urolithiasis in IBD patients may lead to early surgical intervention for asymptomatic stone patients as well as pre-emptive dietary and medical management of stone disease, where possible. Stone prevention protocols tailored to IBD patients, incorporating periodic 24-hour urine metabolic studies, could also reduce the frequency of serious infections, renal damage, and hospitalisation. Future research in this area, such as noninvasive low-cost screening, could result in significant quality improvement and cost savings.
Despite higher rates of UTI, sepsis, and end-organ failure among IBD patients, there was no recorded difference for in-hospital mortality between the two groups of stone-formers. This finding suggests that although patients with IBD are likely to progress to systemic and serious infection more frequently and rapidly than a typical stone patient, the management of this condition, namely decompression of the obstructed urinary tract, is extremely effective [42–45].
Interestingly, a previous population-based study investigating infection-related hospitalisations among IBD patients showed that infection itself was associated with significant mortality (OR, 4.4 fold) but that infections such as pneumonia and Clostridium difficile colitis were much stronger predictors of mortality than UTI among IBD patients [34]. Another study looking at all infected urolithiasis demonstrated that mortality rates have remained stable over time [46].
IBD, extraintestinal manifestation and renal insufficiency
Apart from the intestinal manifestation, there is also a wide range of extraintestinal manifestations, including renal insufficiency (RI) which may sometimes gain greater importance than the underlying disease due to their severity and possible life-threatening consequences [47–49]. They can be divided into reactive manifestations (often associated with inflammatory disease activity) and genuine extraintestinal complications (due to metabolic or anatomical abnormalities caused directly by IBD) [48]. Although some extraintestinal manifestations are more common in CD, they can be found in both entities and add significantly to the burden of the disease [49]. The prevalence of extraintestinal manifestations in IBD varies from 6% to 46% [50–52]. Often joints (arthropathies), skin (erythema nodosum and pyoderma gangrenosum) or eyes are involved, however parenchymatous organs may be affected as well. These manifestations are not frequently encountered and therefore rarely reported; the best known is primary sclerosing cholangitis (mostly linked to UC), a chronic inflammatory condition of the intra- and extrahepatic bile ducts leading to fibrosis and death [50–52]. Other involvement of parenchymatous organs such as kidney or lung receives little mention in the literature. The literature provides little data on renal involvement; so far there are no data available focusing solely on the prevalence of renal insufficiency in IBD.
The first task is to determine the prevalence of RI in IBD and to look for a possible difference between CD and UC. The second one is to investigate if renal impairment might be a complication of the underlying disease or just a side-effect of drugs – at least some of them, mainly cyclosporine, are known to have a nephrotoxic potential – used to treat the disease.
In the study of Primas et al. [38] 2% of the patients with CD developed RI, but none of the patients with UC. A longer duration of CD was associated with a higher frequency of RI in our patients. A longer duration of CD may lead to accumulative damage of the bowel, thus more complications resulting in a greater need for surgeries.
The risk of developing urolithiasis in IBD is 10–100× greater than the risk for general hospital patients [53]. The reasons for that may be volume depletion (leading primarily to uric acid stones) and hyperoxaluria in CD. Small bowel resection leads to both – volume depletion and hyperoxaluria – therefore promoting stone formation and the development of RI. Supporting that, there was a correlation between the length of resected small bowel and the number of interventions due to urolithiasis. Although it would have been interesting, there is suprisingly only limited data on the nature of the stones found in the patients, as the stones are not routinely analysed.
Other causes of renal impairment included amyloidosis, glomerulonephritis, tubulointerstitial nephritis, and nephrolithiasis [54–58]. There are many case reports on amyloidosis, but a review of the literature shows the overall prevalence in IBD to be below 1% [54,55]. Glomerulonephritis has recently emerged as an extraintestinal manifestation and seems to be very rare with about 40 reports in the literature; it appears to be linked to disease activity as renal function improves after remission of IBD [53,56,57]. Tubulointerstitial nephritis seems to be a common clinical feature among IBD patients, manifesting with proteinuria. Lukas et al. [59] in IBD patients on long-term therapy of mesalazin (similar to acetylosalicylic acid) confirmed potential nephrotoxic effect (prevalence 1: 500 treated patients) and recommended routinly performed control of blood urea, creatinine and urine every six months. As with glomerulonephritis there seems to be a correlation with disease activity [53,58]. The emergence of new drugs and the use of more potent drugs earlier in the course of IBD might prevent irreversible damage to the small bowel, therefore reducing the need for surgery. Thus, long-term the prevalence of RI in IBD will hopefully be reduced. In the meantime, it is important to pay attention to older patients with resections in the small bowel and/or urolithiasis and to closely monitor them for signs of RI.
Treatment of urolithiasis in patients with IBD
Patients with a short bowel who develop hyperoxaluria are typically managed by restricting dietary fat and oxalate and by providing an oral calcium supplement, but one must be careful with calcium therapy and monitor it. Excessive calcium can actually increase the CaOx supersaturation in the urine and thus increase the risk of stone formation. Patients with refractory hyperoxaluria may benefit from cholestyramine, which limits bile salt injury to the colon. Bile salts can make the colon more permeable to oxalate and thus facilitate oxalate absorption from the colon.
Rationale for prescribing oral citrate and magnesium to patients with hyperoxaluria, hypocitraturia, and hypomagnesiuria for the prevention of oxalate nephrolithiasis
Hypocitraturia is an important risk factor for CaOx nephrolithiasis [60]. Kato et al. [61] administered potassium-sodium citrate and magnesium oxide to 14 patients with recurrent CaOx stones. After administration of both supplements to the patients with stones, the citrate, magnesium, and potassium levels in 24-hour urine samples increased by 62.1%, 63.3%, and 25.3%, respectively, and oxalate decreased by 66.5%. These authors concluded that the combination of potassium-sodium citrate and magnesium oxide is more effective than either supplement alone in inhibiting the crystallization of CaOx stones by improving the urinary parameters of patients with hypocitraturia and/or hypomagnesiuria. However, in a double-blind, randomized, placebo-controlled trial, there was no significant difference between recurrence rates with 650 or 1,300 mg magnesium oxide daily and the placebo. Another trial reported 391 mg (21 mEq) magnesium daily as a mixed salt, magnesium potassium citrate, reduced calcium stone recurrence by 90%, similar to potassium citrate, but with better gastrointestinal tolerance [62]. Neither magnesium potassium citrate nor potassium-sodium citrate was available in the British National Formulary, therefore potassium citrate mixture was prescribed to this patient.
Importance of nutritionally balanced diet in prevention of urolithiasis
Siener et al. [63] demonstrated that a nutritionally balanced diet significantly reduced stone-forming potential in men and women with CaOx stone disease, although no change occurred in urinary oxalate and magnesium excretion. This patient was prescribed potassium citrate and was advised to adhere to a nutritionally balanced diet avoiding: 1. oxalate-rich food, 2. low fluid intake, and 3. increased intake of protein and alcohol. Following the nutritional intervention with the patient, 24-hour urine oxalate excretion decreased from 0.618 to 0.385 mmol/day; 24-hour urine citrate increased from 0.58 to 1.0 mmol/day.
Biological manipulation of intestinal flora in treatment of hyperoxaluria and oxalate nephrolithiasis
Oxalate is present in many foods and beverages. Bacterial enzymes are required for the intestinal degradation of oxalate in humans [64]. Intestinal oxalate-degrading bacteria are capable of degrading oxalate to CO2 and formate, the latter being absorbed or further metabolized. O. formigenes, an obligate anaerobic microbe normally found in the intestinal tract, contains two enzymes – formyl CoA transferase and oxalyl-coenzyme A decarboxylase – that allow it to utilise oxalate as an energy source, in the process converting oxalate to formate and CO2, as well as a specific oxalate/formate antiporter (Ox1T). The oxalate- -degrading enzyme oxalyl-coenzyme A decarboxylase is also found in Bifidobacterium lactis. Lack of O. formigenes can result in higher absorption of oxalate, leading to an increased risk of CaOx kidney stone formation [65].
Duncan et al. [66] administered O. formigenes by mouth to human volunteers and found a reduction in the amount of oxalate excreted during the six hours immediately following ingestion of an oxalate load (from 3.0 ± 0.6 to 1.9 ± 0.1 mg/h). In the same tests, the ingestion of O. formigenes also decreased the urinary oxalate/creatinine ratio from 45.2 ± 9.9 to 27.0 ± 4. 2 mg/g. Hoppe et al. [67] found the oral application of O. formigenes successful in patients with PH. In one patient with constant intestinal colonization with O. formigenes, urinary oxalate excretion returned to a normal level over time. Other bacteria with possible oxalate-degrading potency are lactic acid bacteria, as well as Enterococcus faecalis and Eubacterium lentum. Although no strain of Lactobacillus acidophilus, L. plantarum, L. brevis, S. thermophilus, or B. infantis expressed the Ox1T gene, the urinary excretion of oxalate, which is a major risk factor for renal stone formation and growth in patients with CaOx urolithiasis, can be reduced with treatment using a high concentration of freeze-dried lactic acid bacteria [68]. Administration of lactic acid bacteria mixture to patients with chronic fat malabsorption, CaOx stones, and hyperoxaluria, resulted in a de- crease in mean urinary oxalate excretion by 19% after one month [69]. Such biological manipulation of the endogenous digestive microflora can be a novel approach for the prevention of urinary stone formation. Therapeutic administration of O. formigenes may ultimately provide the best practical approach for the prevention or alleviation of hyperoxaluria together with potassium citrate supplementation.
Effect of antibiotic therapy on intestinal colonization with O. formigenes
O. formigenes is assumed to be antibiotic-sensitive and repeated antibiotic therapies could eradicate it.
Mittal et al. [70] observed a direct association between antibiotic consumption and absence of O. formigenes in stool samples. However, frequent use of antibiotics may adversely affect intestinal colonization of O. formigenes. In children with cystic fibrosis, prolong- ed and widespread use of antibiotics induced a permanent decolonization of the intestine by O. formigenes. Only 1 of the 43 children with cystic fibrosis who were tested for O. formigenes had normal numbers of O. formigenes in stool samples; this patient with normal colonization by O. formigenes had not been treated with antibiotics [71]. Seven patients who were colonized with O. formigenes had normal urinary oxalate levels, but 19 (53%) of 36 patients who were not colonized with O. formigenes were hyperoxaluric. Absence of O. formigenes from the intestinal tract of children with cystic fibrosis appeared to lead to increased absorption of oxalate, thereby increasing the risk of hyperoxaluria. Such antibiotic induced decolonization of the gut may occur in spinal cord injury patients as well. Troxel et al. [72,73] recommended the use of antibiotics such as penicillin or trimethoprim-sulfamethoxazole instead of quinolones (e. g., ciprofloxacin for urine infection) in CaOx stone-formers, since quinolones reduced the level of O. formigenes in the gut, whereas penicillin or trimethoprim-sulfamethoxazole did not have any effect on the O. formigenes level in the gut.
In future, oral administration of O. for- migenes or lactic acid bacteria may prove to be a promising new therapeutic tool in patients with PH and SH. Lactic acid bacteria are currently classified as nonpathogenic bacteria, which are permitted in food by the U.S. Food and Drug Administration. However, caution may be required when probiotic preparations are used in immunocompromised patients [74,75].
Dietary treatment of hyperoxaluria
Dietary oxalate, its precursors and management [76]
- In human experiments, the intestinal absorption of oxalate is greater on a diet high in oxalate (600 mg) compared to that on a diet poor in oxalate (63 mg), although there is an adaptation with lower rate of absorption if the diet with a high content of oxalate continues for over six weeks.
- A diet rich in oxalate causes a significant increase in urinary oxalate levels.
- A diet low in oxalate is effective in reducing urinary excretion of oxalate and urinary saturation for CaOx with respect to a basal free choice diet.
- In patients with idiopathic calculi an intake of ascorbic acid > 1 g/day is more frequent than in controls. An intake of ascorbic acid may lead to an increase in serum and urinary levels of oxalate by increasing the intestinal absorption and endogenous synthesis. In total parenteral nutrition, an increase of ascorbic acid infusion from 100 to 200 mg induces an increase of oxaluria of about 0.10 mmol/day.
- The increase of oxaluria after oral ingestion of large amounts of ascorbic acid is not easily predictable and has not been confirmed unanimously but it was suggested that patients at risk of kidney stones should not exceed an intake of 500 mg/day.
- Epidemiological studies have shown that the excretion of oxalate is inversely related to a dietary intake of magnesium.
- The rate of intestinal absorption of oxalate, evaluated with [13C2]oxalate in healthy volunteers on a diet containing 800 mg of calcium, ranges from between 2.2% and 18.5%. The intraindividual variation is wide (3.4 ± 1.7%).
- The intestinal absorption of radioactive [13C2]oxalate in a diet containing 1,200 mg of calcium (diet + supplements of calcium citrate and calcium carbonate) is 2%, but it increases to 17% at 200 mg of dietary calcium. The increase is linear with a decrease of dietary calcium, but there is no further reduction of intestinal absorption of oxalate when dietary calcium increases > 1,200 mg.
- In healthy subjects and calcium renal stone-formers, a low-calcium diet causes a significant decrease in the levels of urinary calcium but increases urinary excretion of oxalate and the risk of renal stone formation.
- In idiopathic renal calcium stone-formers with hypercalciuria, a low-calcium diet increases urinary oxalate excretion more than in normocalciuric. On low-calcium diet urinary oxalate excretion is related to the degree of intestinal absorption of calcium.
- In renal stone-formers with idiopathic hypercalciuria, a high-calcium diet (900–1,070 mg/day) decreases the excretion of oxalate, the oxalate/creatinine ratio and the lithogenic risk in comparison with a normal-calcium diet (700 mg/day).
- In CaOx renal stone patients with hyperoxaluria, the addition of supplements of calcium citrate to the low- oxalate diet did not result in a greater decrease of urinary excretion of oxalate than diet alone, although the supersaturation for CaOx decreases more.
- Vegetarians have higher urinary oxalate levels than controls on a free-choice mixed mediterranean diet with significantly higher calcium/oxalate ratio resulting from a higher intake of oxalate and increased fractional intestinal absorption of oxalate.
- In vegetarians the risk of CaOx crystallization is not decreased (increase in urinary pH, citrate and magnesium excretion and decline in calcium excretion, but increase in urinary oxalate by 30%).
- In patients with hyperoxaluria (> 40 mg), a diet rich in fruits, vegetables, whole grains and low-fat dairy products and low in total fat and saturated fat, cholesterol, refined carbohydrates and sweets and meat, which is recommended for patients with hypertension (DASH – dietary approaches to stop hypertension) results in a slight increase in urinary excretion of oxalate compared with a diet low in oxalates, but decreases the supersaturation with respect to CaOx due to the concomitant increase in the excretion of magnesium and citrate and increase of urinary pH.
- In normal healthy subjects the abolition of fruits and vegetables induces a slight increase of CaOx and calcium phosphate saturation as it reduces excretion of citrate, magnesium, potassium and also oxalate, while increasing urinary excretion of calcium and ammonium.
- In hypocitraturic calcium renal stone-formers, adding fruits and vegetables increases excretion of magnesium and citrate, urinary pH and urinary volume, without changing the excretion of oxalate and calcium. The result is a significant reduction of lithogenic risk for calcium salts and for uric acid.
- A moderate intake of glycine (4.5 g daily) or protein (50 g daily, 50% animal protein) has no effect on serum or urinary oxalate.
- A diet very rich in meat (700 g of meat or fish daily, 2.26 g of protein/kg daily) increases urinary oxalate in approximately one third of patients with calcium nephrolithiasis, with an average increase of 73 μmol/24 hours whereas no change is observed in normal subjects. Patients with mild hyperoxaluria have a more substantial increase of urinary oxalate (+ 100 μmol). The mechanism of sensitivity is not clear, but does not involve a deficit in vitamin B6.
- The effect of a reduction in dietary protein on urinary excretion of oxalate is controversial. Changing calcium stone-formers from a high to a low animal protein intake causes no variation in urolithiasis. In renal stone-formers with idiopathic hypercalciuria, a moderate restriction of protein intake causes a reduction of urinary calcium, urate and oxalate and improves the profile of lithogenic patients. A diet with a reduced intake of protein (< 93 g) and salt (50 mmol) results in a significant reduction of urinary oxalate excretion and CaOx product.
- In hypercalciuric renal stone-formers, the addition of 30 g of dietary fibre as unprocessed wheat bran to a low-calcium and low-oxalate diet results in a 23.5% decrease of urinary calcium with respect to the 5.6% decrease obtained with the diet alone whereas the addition of fibre results in a 3.9% decrease in urinary oxalate compared to the 21.4% on diet alone.
- The administration of pyridoxine in oral doses of 250–500 mg daily to both normo- or hyperoxaluric calcium renal stone-formers decreases urinary oxalate excretion.
- The administration of lactobacilli has proved to be effective in reducing the levels of urinary excretion of oxalate in renal calcium stone patients with idiopathic hyperoxaluria and calcium (> 40 mg/day), in healthy subjects consuming a high oxalate diet and in renal stone formers without hyperoxaluria on a diet rich in oxalate.
- Hyperoxaluric patients show higher lipid intake and lower glucidic and calcium intake.
Dietary counselling according to the Recommended Dietary Allowance results in a reduction of intake of total protein, animal protein, fat, and carbohydrates, and it is associated with a reduction in the excretion of oxalate. The effect of general dietary measures (balanced diet, specific diet) on urinary oxalate has not always proven effective in reducing urinary oxalate. The dietary approach is particularly recommended after extracorporeal shock wave lithotripsy.
Example of medical treatment towards prevention of recurrent kidney stone
This patient had a short bowel and hyperoxaluria; a sample of stool tested negative for O. formigenes.
He was therefore advised to avoid oxalate-rich food as well as fatty food, and adhere to a nutritionally balanced diet, which included recommended levels of calcium and bio yogurt containing Lactobacillus acidophilus, Streptococ- cus thermophilus, and bifidobacterium. He was also prescribed potassium citrate mixture 10 mL, 3× daily, well diluted with water, by mouth. The composition of this oral solution was potassium citrate, 3 g; citric acid monohydrate, 500 mg; syrup, 2.5 mL; quillaia tincture, 0.1 mL; lemon spirit, 0.05 mL; double-strength chloroform water, 3 mL; water to 10 mL. This mixture contained about 28 mmol K+/10 mL. Two months later, 24-hour urinary oxalate decreased from 0.618 to 0.411 mmol/day; 24-hour urine citrate increased from 0.58 to 1.10 mmol/day. Six months later, an oxalate absorption test was performed.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“
Submitted/ Doručeno: 20. 11. 2015
Accepted/ Přijato: 27. 11. 2015
Prof. MU Dr. Vladimír Teplan, DrSc
IBD Clinical and Research Centre,
ISCARE Lighthouse
Jankovcova 1569/ 2c
170 04 Praha 7
vladimir.teplan@seznam.cz
Literature
1. Romanko I, Lukas M, Bortlik M. New approaches in the follow-up of patients suffering from inflammatory bowel disease. Gastroent Hepatol 2015; 69 (5): 441–448. doi: 10.14735/amgh2015441.
2. Manganiotis AN, Banner MP, Malkowicz SB. Urologic complications of Crohn’s dis- ease. Surg Clin North Am 2001; 81 (1): 197–215.
3. Banner MP. Genitourinary complications of inflammatory bowel disease. Radiol Clin North Am 1987; 25 (1): 199–209.
4. Knudsen L, Marcussen H, Fleckenstein P et al. Urolithiasis in chronic inflammatory bowel disease. Scand J Gastroenterol 1978; 13 (4): 433–436.
5. McLeod RS, Churchill DN. Ultrolithiasis complicating inflammatory bowel disease. J Urol 1993; 148 (2): 974–978.
6. Maratka Z, Nedbal J. Urolithiasis as a complication of the surgical treatment of ulcerative colitis. Gut 1964; 5: 214–217.
7. Worcester EM. Stones due to bowel disease. In: Coe FL, Favus MJ, Pak CYC et al (eds). Kidney stones: medical and surgical management. Philadelphia: Lippincott-Raven 1996: 883–904.
8. Deren JJ, Porush JG, Levitt MF et al. Nephrolithiasisas a complication of ulcerative colitis and regional enteritis. Ann Intern Med 1962; 56: 843–853.
9. Robertson WG, Peacock M, Baker M et al. Studies on the prevalence and epidemiology of urinary stone disease in men in Leeds. Br J Urol 1983; 55 (6): 595–598.
10. Curhan GC, Rimm EB, Willett WC et al. Regional variation in nephrolithiasis incidence and prevalence among United States men. J Urol 1994; 151 (4): 838–841.
11. Soucie JM, Thun MJ, Coates RJ. Demographic and geographic variability of kidney stones in the United States. Kidney Int 1994; 46 (3): 893–899.
12. Parks JH, Worcester EM, O’Connor RC et al. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003; 63 (1): 255–265.
13. McConnell N, Campbell S, Gillanders I et al. Risk factors for developing renal stones in inflammatory bowel disease. BJU Int 2002; 89: 835–841.
14. Ishii G, Nakajima K, Tanaka N et al. Clinical evaluation of urolithiasis in Crohn’s disease. Int J Urol 2009; 16 (5): 477–480. doi: 10.1111/j.1442-2042.2009.02285.x.
15. Gustavsson A, Halfvarson J, Magnuson A et al. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior tothe immunosuppressive treatment era. Am J Gastroenterol 2007; 102 (11): 2513–2519.
16. Filippi J, Allen PB, Hebuterne X et al. Does anti-TNF therapy reduce the requirement for surgery in ulcerative colitis? A systematic review. Curr Drug Targets 2011; 12 (10): 1440–1447.
17. Shen B, Remzi FH, Oikonomou IK et al. Risk factors for low bone mass in patients with ulcerative colitis following ileal pouch-anal anastomosis. Am J Gastroenterol 2009; 104 (3): 639–646. doi: 10.1038/ajg.2008.78.
18. Oikonomou IK, Fazio VW, Remzi FH et al. Risk factors for anemia in patients with ileal pouch-anal anastomosis. Dis Colon Rectum 2007; 50 (1): 69–74.
19. Kuisma J, Luukkonen P, Jarvinen H et al. Risk of osteopenia after proctocolectomy and ileal pouch-anal anastomosis for ulcerative colitis. Scand J Gastroenterol 2002; 37 (2): 171–176.
20. Bennett RC, Hughes ES. Urinary calculi and ulcerative colitis. Br Med J 1972; 2 (5812): 494–496.
21. Knudsen L, Marcussen H, Fleckenstein P et al. Urolithiasis in chronic inflammatory bowel disease. Scand J Gastroenterol 1978; 13 (4): 433–436.
22. Gelzayd EA, Breuer RI, Kirsner JB. Nephrolithiasis in inflammatory bowel disease. Am J Dig Dis 1968; 13 (12): 1027–1034.
23. Kennedy HJ, Al-Dujaili EA, Edwards CR et al. Water and electrolyte balance in subjects with a permanent ileostomy. Gut 1983; 24 (8): 702–705.
24. Watts RW. Primary hyperoxaluria type I. QJM 1994; 87 (10): 593–600.
25. Robijn S, Hoppe B, Vervat BA et al. Hyperoxaluria: a gut-kidney axis? Kidney Int 2011; 80 (11): 1146–1158. doi: 10.1038/ki.2011. 287.
26. Streit J, Tran-Ho L, Konigsberger E. Solubility of the three calcium oxalate hydrates in sodium chloride solutions and urine-like liquors. Monatsh Chem Chem Mon 1998; 129: 1225–1236.
27. Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am 2002; 31 (4): 927–949.
28. Earnest DL. Enteric hyperoxaluria. Adv Intern Med 1979; 24: 407–427.
29. Williams HE. Oxalic acid and the hyperoxaluric syndromes. Kidney Int 1978; 13 (5): 410–417.
30. Molodecky NA, Soon IS, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142 (1): 46–54. doi: 10.1053/j.gastro.2011.10.001.
31. Lichtenstein GR, Feagan BG, Cohen RD et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012; 107 (9): 1409–1422. doi: 10.1038/ajg.2012.218.
32. Aberra FN, Lichtenstein GR. Methods to avoid infections in patients with inflammatory bowel disease. Inflamm Bowel Dis 2005; 11 (7): 685–695.
33. Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis 2008; 14 (8): 1105–1111. doi: 10.1002/ibd.20429.
34. Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohn Colitis 2013; 7 (2): 107–112. doi: 10.1016/j.crohns.2012.02.015.
35. Naganuma M, Kunisaki R, Yoshimura N et al. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol 2012; 48 (5): 595–600. doi: 10.1007/s00535-012-0686-9.
36. Peyrin-Biroulet L, Pillot C, Oussalah A et al. Urinary tract infections in hospitalized inflammatory bowel disease patients: a 10-year experience. Inflamm Bowel Dis 2012; 18 (4): 697–702. doi: 10.1002/ibd.21 777.
37. Pardi DS, Tremaine WJ, Sandborn WJ et al. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol 1998; 93 (4): 504–514.
38. Primas C, Novacek G, Schweiger K et al. Renal insufficiency in IBD – prevalence and possible pathogenetic aspects. J Crohn Colitis 2013; 7 (12): 630–634. doi: 10.1016/j.crohns.2013.05.001.
39. Cury D, Moss A, Schor N. Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis 2013; 6: 139–142. doi: 10.2147/IJNRD.S45466.
40. Huang V, Mishra R, Thanabalan R et al. Patient awareness of extraintestinal manifestations of inflammatory bowel disease. J Crohn Colitis 2013; 7 (8): 318–324. doi: 10.1016/j.crohns.2012.11.008.
41. Kornbluth A, Hayes M, Feldman S et al. Do guidelines matter? Implementation of the ACG and AGA osteoporosis screening guidelines in inflammatory bowel disease (IBD) patients who meet the guidelines’ criteria. Am J Gastroenterol 2006; 101 (7): 1546–1550.
42. Mokhmalji H, Braun PM, Martinez Portillo FJ et al. Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: a prospective, randomized clinical trial. J Urol 2001; 165 (4): 1088–1092.
43. Pearle MS, Pierce H, Miller GL et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol 1998; 160 (4): 1260–1264.
44. Wenzler DL, Kim SP, Rosevear HM et al. Success of ureteral stents for intrinsic ureteral obstruction. J Endourol 2008; 22 (2): 295–300. doi: 10.1089/end.2007.0201.
45. Yossepowitch O, Lifshitz DA, Dekel Y et al. Predicting the success of retrograde stenting for managing ureteral obstruction. J Urol 2001; 166 (5): 1746–1749.
46. Sammon JD, Ghani KR, Karakiewicz PI et al. Temporal trends, practice patterns, and treatment outcomes for infected upper urinary tract stones in the United States. Eur Urol 2013; 64 (1): 85–92.
47. Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med Mar 2010; 42 (2): 97–114. doi: 10.3109/07853890903559724.
48. Danese S, Semeraro S, Papa A et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol 2005; 11 (46): 7227–7236.
49. Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol 2006; 14 (30): 4819–4831.
50. Bernstein CN, Blanchard JF, Rawsthorne P et al. The prevalence of extraintestinal dis- eases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 1996; 96 (4): 1116–1122.
51. Ricart E, Panaccione R, Loftus EV Jr et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2004; 10 (3): 207–214.
52. Mendoza JL, Lana R, Taxonera C et al. Extraintestinal manifestations in inflammatory bowel disease: differences between Crohn’s disease and ulcerative colitis. Med Clin (Barc) 2005; 125 (8): 297–300.
53. Pardi DS, Tremaine WJ, Sandborn WJ et al. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol 1998; 93 (4): 504–514.
54. Serra I, Oller B, Mañosa M et al. Systemic amyloidosis in inflammatory bowel disease: retrospective study on its prevalence, clinical presentation and outcome. J Crohns Colitis Sep 2010; 4 (3): 269–274. doi: 10.1016/j.crohns.2009.11.009.
55. Basturk T, Ozagari A, Ozturk T et al. Crohn‘s disease and secondary amyloidosis: early complication? A case report and review of the literature. J Ren Care 2009; 35 (3): 147–150. doi: 10.1111/j.1755-6686.2009.00106.x.
56. Peeters AJ, van den Wall Bake AW, Daha MR et al. Inflammatory bowel disease and ankylosing spondylitis associated with cutaneous vasculitis, glomerulonephritis, and circulating IgA immune complexes. Ann Rheum Dis 1990; 49 (8): 638–640.
57. Shaer AJ, Stewart LR, Cheek DE et al. IgA antiglomerular basement membrane nephritis associated with Crohn‘s disease: a case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis 2003; 41 (5): 1097–1109.
58. Kreisel W, Wolf LM, Grotz W et al. Renal tubular damage: an extraintestinal manifestation of chronic inflammatory bowel disease. Eur J Gastroenterol Hepatol 1996; 8 (5): 461–468.
59. Lukas M, Bortlík M, Novotný A et al. Nefrotoxicita mesalazinu při dlouhodobé léčbě ulcerózní kolitidy a Crohnovy nemoci. Čes a Slov Gastroent 1999; 53 (5): 135–139.
60. Pak CY. Medical management of urinary stone disease. Nephron Clin Pract 2004; 98 (2): c49–c53.
61. Kato Y, Yamaguchi S, Yachiku S et al. Changes in urinary parameters after oral administration of potassium-sodium citrate and magnesium oxide to prevent urolithiasis. Urology 2004; 63 (1): 7–11.
62. Massey L. Magnesium therapy for nephrolithiasis. Magnes Res 2005; 18 (2): 123–126.
63. Siener R, Schade N, Nocolay C et al. The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 2005; 173 (5): 1601–1605.
64. Stewart CS, Duncan SH, Cave DR. Oxalobacter formigenes and its role in oxalate metabolism in the human gut. FEMS Microbiol Lett 2004; 230 (1): 1–7.
65. Delvecchio FC, Preminger GM. Medical management of stone disease. Curr Opin Urol 2003; 13 (3): 229–233.
66. Duncan SH, Richardson AJ, Kaul P et al. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol 2002; 68 (8): 3841–3847.
67. Hoppe B, von Unruh G, Laube N et al. Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res 2005; 33 (5): 372–375.
68. Campieri C, Campieri M, Bertuzzi V et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 2001; 60 (3): 1097–1105.
69. Lieske JC, Goldfarb DS, De Simone C et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int 2005; 68 (3): 1244–1249.
70. Mittal RD, Kumar R, Bid HK et al. Effect of antibiotics on Oxalobacter formigenes colonization of human gastrointestinal tract. J Endourol 2005; 19 (1): 102–106.
71. Sidhu H, Hoppe B, Hesse A et al. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet 1998; 352 (9133): 1026–1029.
72.Troxel SA, Sidhu H, Kaul P et al. Intestinal Oxalobater formigenes colonisation in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol 2003; 17 (3): 173–176.
73. Hoppe B, Leumann E, von Unruh G et al. Diagnostic and therapeutic approaches in patients with secondary hyperoxaluria. Front Biosci 2003; 8: e437–e443.
74. Cannon JP, Lee TA, Bolanos JT et al. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 2005; 24 (1): 31–40.
75.Vaidyanathan S, von Unruh GE, Watson ID et al. Hyperoxaluria, hypocitraturia, hypomagnesiuria, and lack of intestinal colonisation by Oxalobacter formigenes in a cervical spinal cor injury patients with suprapublic cystostomy, short bowel, and nephrolithiasis. Scienfific World J 2006; 6 (6): 2403–2410.
76. Prezioso D, Strazzullo P, Lotti T et al. Dietary tratment of urinary risk factors for renal stone formation. A review of CLU Working Group. Arch Ital Urol Androl 2015; 87 (2): 105–120. doi: 10.4081/aiua.2015.2.105.
77. Nazzal L, Puri S, Goldfarb DS. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant 2015; pii: gfv005.