Hepatic parenchyma changes in obese paediatric patients
Marek Pršo1, Linda Pršová1, Zuzana Havlíčeková1, Zuzana Michnová1, Marek Kozár2, Kristián Pršo3, Peter Bánovčin jr.4, Ľubomír Skladaný5, Peter Bánovčin1
+ Affiliation
Summary
Rationale: Non-alcoholic fatty liver disease (NAFLD) is emerging clinical issue in childhood and adolescent age. Present knowledge of NAFLD suggests an important role of genetic and environmental risk factors in the pathogenesis of the disease. Most of the patients are obese, however, NAFLD also occurs in the non-obese group and interestingly, in obese individuals it may be absent. The transabdominal ultrasound examination is the most widely used imaging method for NAFLD screening. Aim: The aim of the study was to assess the impact of paediatric obesity as the main risk factor in NAFLD development. Materials and methods: The degree of steatosis (represented by hepatorenal index) and hepatic parenchyma stiffness (represented by fibrosis liver index) were quantitatively evaluated using ultrasound device in a total of 240 paediatric and adolescent patients divided in subgroups according to age and weight criteria. Results from ultrasound examination were subsequently correlated with anthropometric and laboratory parameters. Results: Hepatorenal index (HRI) and liver fibrosis index (LFI) in healthy term neonates with normal birth weight was significantly lower compared to the control group of healthy normal weight children aged 10–18 years (p <0.001). We did not observe an effect of gender on changes in HRI (p = 0.332) and LFI (p = 0.339) in teenage and adolescent controls. Regardless of gender, normal HRI values in paediatric heathy control group ranged from 1.02–1.23 (10th–90th percentile). The group of obese children aged 10–18 years had HRI and LFI values significantly higher in contrast with healthy normal weight controls. Obese individuals had liver stiffness proportional to BMI (p = 0.005, rs = 0.310), however, the steatosis degree remained unchanged (p = 0.357). Hepatic parenchyma stiffness also increased with waist circumference gain in corpulent patients (p <0.01). Conclusion: Results of this study point to a significant association of obesity and NAFLD in paediatric population. The assessment of HRI and liver stiffness using ultrasound methods have been employed in the diagnosis of early stages of hepatic changes in obese children and adolescent patients at risk of NAFLD development. In addition to the early detection in these changes, ultrasound determination enables non-invasive and real-time assessment of dynamics of the disease and the effect of the administered therapy, which improves control over the disease.
Keywords
non-alcoholic fatty liver disease, obesity, hepatorenal index, real-time elastography, body roundness index, paediatric age, adolescent age
Introduction
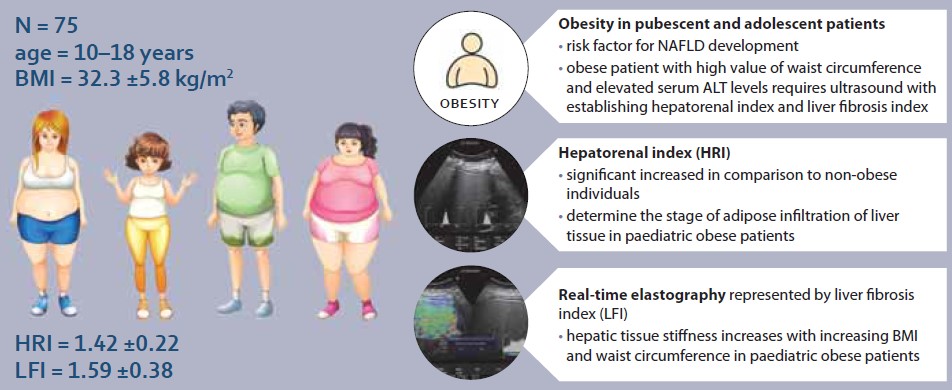
Obesity is an increasing global issue in adults as well as in childhood and adolescent age. Incidence in paediatric population in countries of Central Europe is reported to range from 14.4–19.2% and 11.8–17.6% for male and female, respectively [1]. It is associated with development of several later complications, mainly cardiovascular diseases, type 2 diabetes mellitus, orthopaedic problems and mental health affection with loss of self-confidence [2]. In addition, obesity is also the main risk factor for the non-alcoholic fatty liver disease (NAFLD) development, which can progress to advanced stages of liver disease. The ultrasound quantification of steatosis represents modern diagnostic approach with capability of accurate disease stage determination. The degree of hepatic steatosis and its stiffness can be evaluated in real-time elastography using ultrasound device with data processing software. However, cut-off values for hepatorenal index (HRI) determining specific degree of steatosis are established for the adult population and, therefore, their application on paediatric population is debatable.
Aim
The aim of the study was the ultrasound evaluation of hepatic condition in group of obese children and adolescents aged between 10–18 with the risk of NAFLD development. Hepatic ultrasound parameters were assessed also in the group of the same age probands with normal weight, the group of non-obese NAFLD patients and in the group healthy full-term neonates. Obtained ultrasound parameters were correlated with laboratory and anthropometric measurements in the group of corpulent patients.
Materials and methods
Group of probands
Clinical study was conducted at the Clinic of Children and Adolescents of the Martin University Hospital from September 2017 to March 2018. A condition for the study inclusion besides selection criteria meeting was also the signed informed consent by the patient or legal representative. A total of 240 individuals were enrolled in the study, divided into 3 group. The first group included 61 healthy full-term newborns with presumed physiological ultrasonographic findings. The second group enrolled 104 normal BMI healthy individuals aged between 10–18 and, thus, likely without liver pathology. Finally, the third group consisted of 75 patients of the same age range as patients in group two, but with exogenous obesity. Patients with variously elevated BMI values from the third group were subsequently stratified into two sub-groups (Obesity Class I and II) considering age, gender and BMI percentile according to International Obesity Task Force (IOTF) [3]. We excluded several patients from the study group due to more serious medical problems, namely viral liver infections, excessive alcohol consumption, anamnesis of parenteral nutrition, use of drugs associated with the development of steatosis, patients with autoimmune and metabolic liver diseases.
Anthropometric and laboratory parameters
The following anthropometric measurements were performed on all subjects. The height was determined to the nearest centimeter with rounding to the whole number. The weight rounded to whole kilograms was assessed before the examination in the post-absorptive state and after urination. All subjects wore only light underwear and were barefoot during weighting. Body mass index was calculated as the ratio of weight in kilograms to the square of the body height in meters (kg/m2). The waist circumference was measured according to the World Health Organisation’s (WHO) general recommendation [2]. In addition to BMI, Body Roundness Index (BRI) was calculated for each subject. BRI considers the waist circumference and height of the patient in meters and is determined according to the following formula [4]:
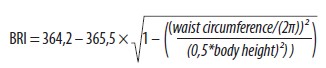
The venous blood was drawn after at least 12 hours starving on the day of ultrasonographic examination in the group of obese patients. The biochemical analyser Olympus AU640/AU700 (Beckman-Coulter, USA) was employed for laboratory parameters determination with the exception of vitamin D. Blood glucose levels were determined using enzymatic photometry in mmol/L units. Similarly, for the evaluation of serum triacylglycerols (TAG), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and total cholesterol in mmol/L the enzymatic photometry was selected. The liver function tests including serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl aminotransferase (GGT) and alkaline phosphatase (ALP) were assessed photometrically in μkat/L units. The total serum bilirubin in mmol/L was evaluated by modified Ehrlich diazo method. For serum vitamin D determination in μg/L, the fully automated electro-chemiluminescent immunoassay Cobas e 411 (Roche, Switzerland) was used.
Ultrasonographic parameters
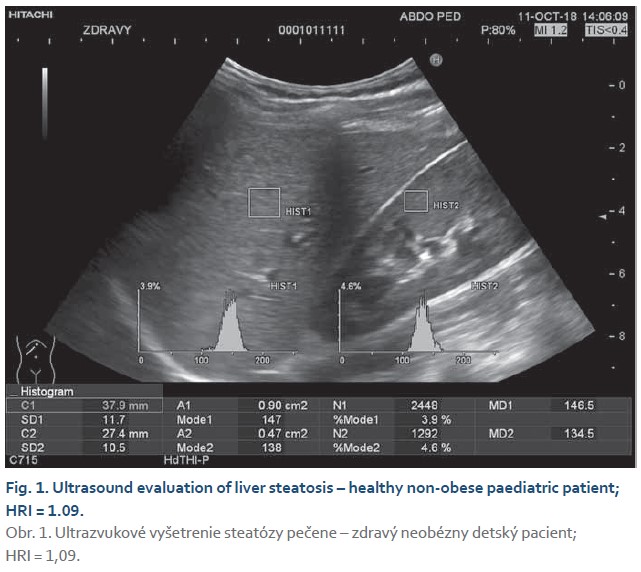
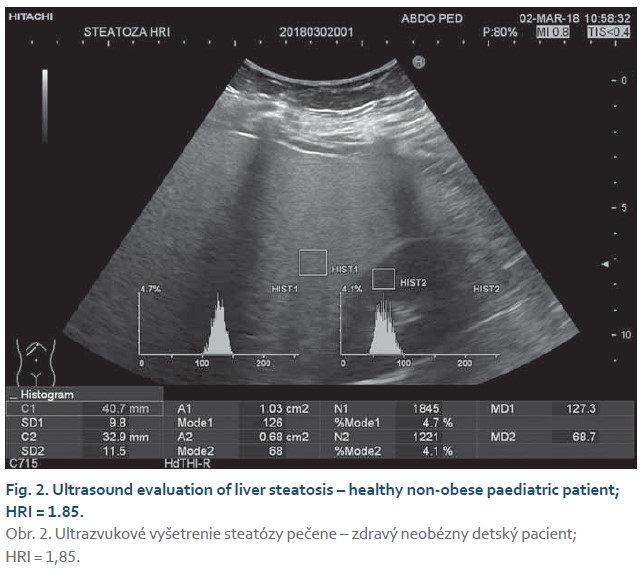
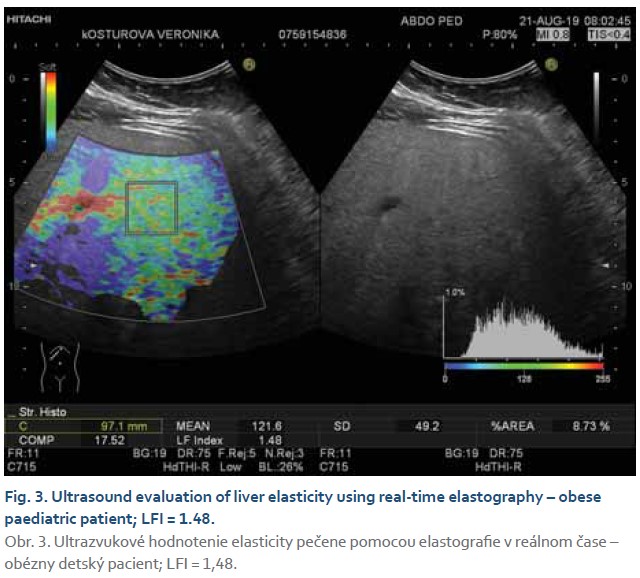
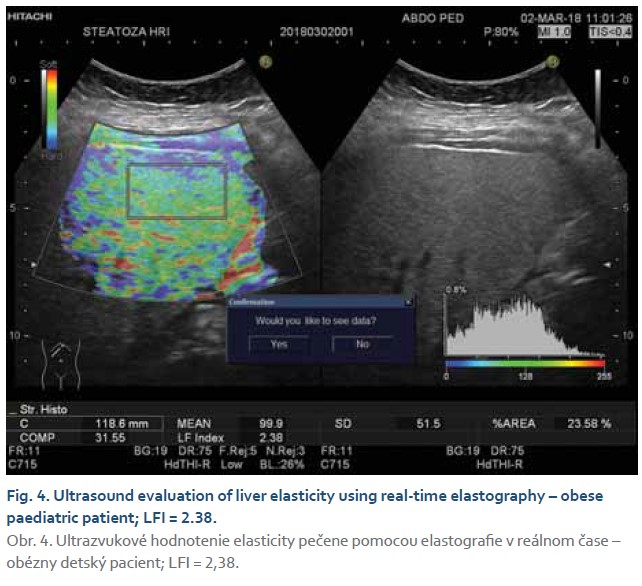
The steatosis degree was appraised using histogram software functionality of the ultrasound device (Hitachi Preirus) based on measured HRI values. Simultaneous capture of liver tissue and right kidney image was obtained using convex ultrasound probe. With a uniform setting of the basic echogenicity, a histogram of the liver and the cortex of the right kidney was realized at the same depth (Fig. 1, 2). The HRI values were obtained by ratios of liver and kidney echogenicity medians. Steatosis is excluded if the HRI values were less than 1.2. Within the HRI range of 1.2–1.5 the non-significant steatosis is present affecting less than 5% of hepatocytes. Significant steatosis corresponding to histological correlate affecting more than 5% of hepatocytes is recognised with 100% sensitivity and 91% specificity at the HRI values ≥1.5. Steatosis extent in more than 25% and 60% of hepatocytes is observed at HRI values greater than 1.89 and 2.23, respectively. However, mentioned cut-off values were set out for adults with tissue biopsy verified steatosis [5]. Determination of liver tissue elasticity was carried out using the same ultrasound device with real-time elastography software. According to the general recommendation for elasticity measurements a convex ultrasound probe was attached to the 5–7 intercostal space in the mid-axillary line. For LFI determination, the most suitable area of interest was chosen from displayed liver elasticity map i.e., without the presence of blood vessels and main bile ducts at least in 1 cm depth below the liver capsule (Fig. 3, 4). The value of tissue elasticity was calculated by software and was expressed as the LFI. Final LFI was calculated as an average of three measurements.
Statistics
The statistical software SYSTAT (Systat Software Inc., USA) was used for data processing and analysis. Gaussian/non-Gaussian data distribution was determined using the Shapiro-Wilk test. One-factor analysis of variance (ANOVA) with post hoc Fischer‘s LSD (least significance difference) test for Gaussian distribution parameters was used for individual intergroup comparisons. Contrary, for non- Gaussian data distribution Kuskal-Wallis test with subsequent Mann-Whitney analysis was used. Gender differences were evaluated using the Student’s T-test for independent samples with Gaussian distribution, and the Mann-Whitney test for parameters with non-Gaussian distribution. Spearman’s correlation Test was used to analyse relationships between variables. The results are presented as average ±SD (for variables with Gaussian distribution) or median and interquartile range (for non-Gaussian distribution of variables). Values of p <0.05 were considered statistically significant.
Results
Baseline cohort characteristics
The study cohort consisted of 240 subjects divided into 3 groups. The first group included 61 healthy term neonates, of which 29 were female (48%) and 32 were male (52%). Average birthweight within this group was 3,379 ±417 grams. Average maternal weight gain during pregnancy was 14.9 ±5.3 kg. The second group consisted of 104 healthy subjects aged 10–18 years of life, including 44 males (42%) and 60 females (58%). All patients had normal BMI values for their age group, with the average BMI being 20.3 ±3.3 kg/m2 (Tab. 1). The third group included 75 paediatric patients with exogenic obesity (48 boys and 27 girls) (Tab. 2).
Evaluation of ultrasonographic parameters
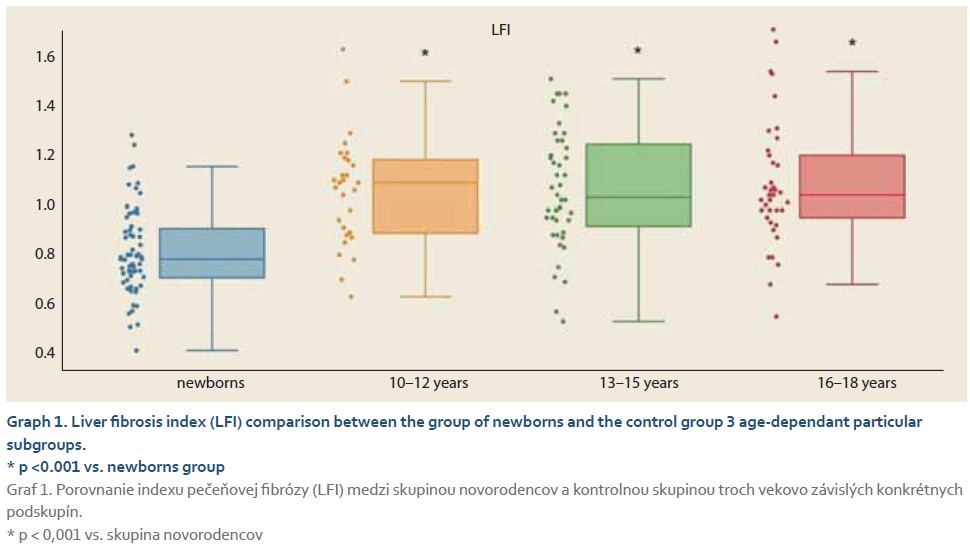
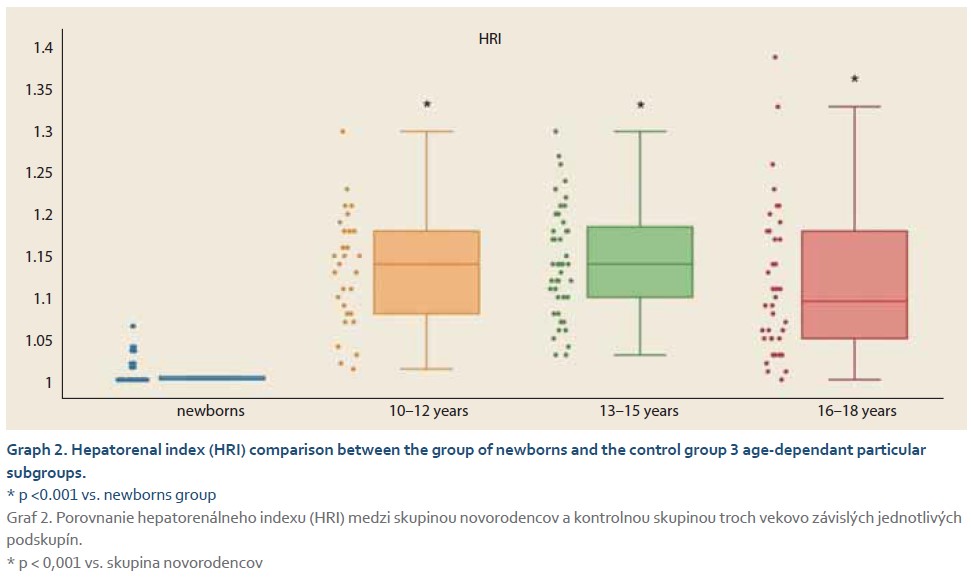
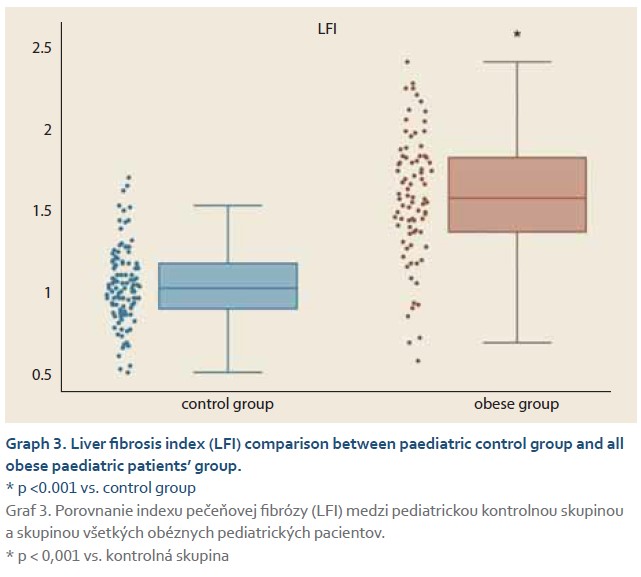
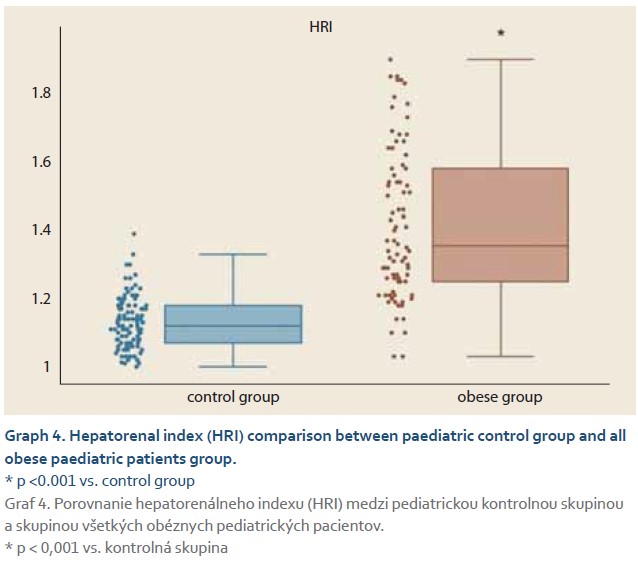
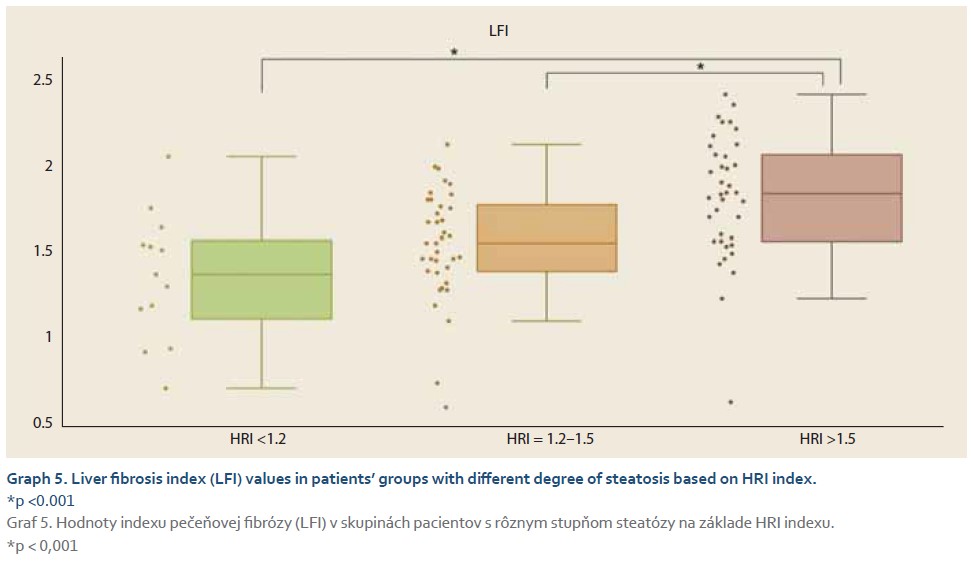
The average elasticity value in neonates expressed by the liver fibrosis index (LFI) was 0.44 ±0.16. The ratio of echogenicity histograms for liver parenchyma and right kidney cortex – the hepatorenal index (HRI) – was 1.01 ±0.02. In the control group of healthy subjects aged 10–18 years and normal weight average values of LFI were 1.06 ±0.23 and average HRI was 1.13 ±0.08. The control group was subsequently divided into 3 subgroups, 10–13-year-olds, 14–16-year-olds and 17–18-year-olds. Comparing the values of LFI and HRI measured by ultrasonography we noted significant differences in both parameters between the neonatal cohort and the control group (p <0.001 for both parameters). No significant differences in ultrasonographic parameters measured within particular subgroups of our control group were observed (Graph 1, 2). In the group of all obese paediatric patients (N = 75) median LFI was 1.58 (1.38–1.83) and median HRI 1.36 (1.25–1.58). Using the Kruskal-Wallis test we noted a significant increase in HRI values in the group of all obese patients in comparison with the control group (x2 = 86.81; p <0.001); their values of HRI were placed within the range of non-significant steatosis (“pre-steatosis”) [5]. The Kruskal-Wallis test also confirmed a significant elevation of LFI values within the group of obese patients (x2 = 109.22; p <0.001) compared to the control group (Graph 3, 4). In both subgroups, sex had no statistically significant influence on LFI or HRI changes. After dividing the group of obese paediatric patients based on their HRI determined via ultrasonography, we have observed a significant decrease of liver elasticity in the subgroup of patients with steatosis diagnosed via ultrasound (HRI ≥1.5), compared to the subgroup of obese patients with non-significant steatosis (HRI = 1.2–1.49) and the group of patients without steatosis (HRI <1.2). There was no significant difference in liver elasticity values between the group of patients with non-significant steatosis and those without steatosis (Graph 5).
Evaluation of laboratory parameters
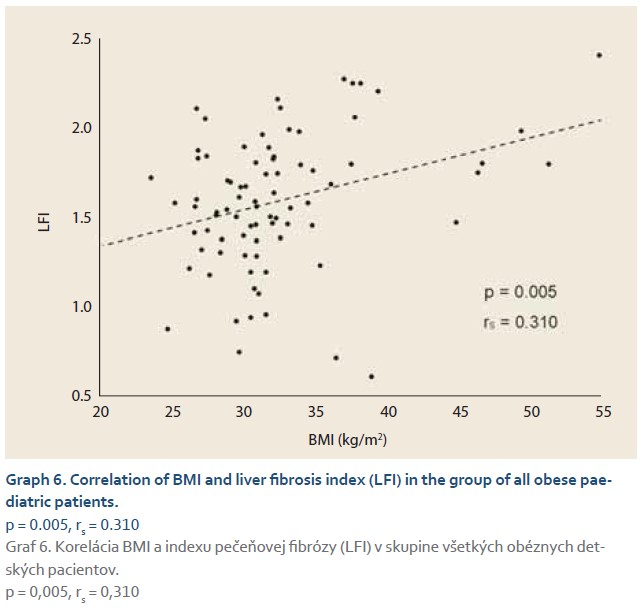
Using the Spearman correlation test we evaluated correlations of ultrasonographic and laboratory parameters within the group of all obese patients (N = 75). In this cohort LFI values were positively correlated with BMI (rs = 0.310; p = 0.005) (Graph 6), BRI (rs = 0.340; p = 0.003), LDL (rs = 0.371; p = 0.001) and total serum cholesterol levels (rs = 0.287; p = 0.001). No statistically significant correlation between the levels of other markers of lipid metabolism, hepatic enzymes and changes in liver elasticity was observed. HRI values were positively correlated with levels of hepatic enzymes AST (rs = 0.265; p = 0.022), ALT (rs = 0.365; p = 0.001), ALP (rs = 0.364; p = 0.001), also with markers of lipid metabolism such as levels of TAG (rs = 0.256; p = 0.027), LDL (rs = 0.300; p = 0.001) and total serum cholesterol (rs = 0.266; p = 0.021). GGT and other laboratory parameters (bilirubin, HDL, serum glucose) did not show a significant correlation with HRI.
Discussion
In the last two decades, NAFLD in paediatric populations has presented a pressing healthcare issue. In developed countries the prevalence of NAFLD reaches 9.6% within the general paediatric population and is currently on the rise in developing countries as well [6]. NAFLD is therefore considered to be the most frequent case of chronic liver disease in paediatric patients. A substantial portion of NAFLD patients are obese and its prevalence increases in morbidly obese patients [6]. In our study we have confirmed a significant association between obesity with NAFLD in a cohort of pubescent and adolescent paediatric patients. Ultrasonographic examination revealed significantly greater stage of liver steatosis in patients with first and second grade obesity, compared to healthy children with normal weight. In the group of patients with second grade obesity we noted a higher grade of steatosis seen via ultrasonographic imaging than in the group of patients with first grade obesity, these values however did not show statistical significance. Our results are in concordance with the conclusions reached by Turkish authors Akcam et al [7] in their work dealing with scoring steatosis in pubescent and prepubescent obese patients using ultrasonography. Several parameters such as hepatorenal echo contrast, deep attenuation and vascular blurring defined by Hamaguchi et al. [8] were evaluated. Their results indicate that NAFLD prevalence was higher in a group of pubescent obese paediatric patients with higher BMI, than in the group of prepubescent obese patients [7]. Using ultrasound, we confirmed that the stage of liver steatosis increases with increasing values of waist circumference in obese patients. A study by Brazilian authors Duarte and Silva [9] showed significantly higher values of waist circumference in obese paediatric patients with liver steatosis [9]. In paediatrics, waist circumference alone can potentially serve as an anthropometric predictor of increased risk for insulin resistance, metabolic syndrome and therefore NAFLD as well [10]. Increase in waist circumference is also associated with higher risk of developing hepatic fibrosis [11]. While evaluating body rounding index (BRI), we noted higher BRI values in obese paediatric patients compared to the control group of healthy subjects. Obese patients with higher BRI had significantly lower hepatic elasticity in comparison with lower BRI patients. The grade of steatosis determined by HRI was similarly elevated in both groups with no significant difference between them. High values of BRI could therefore be associated with lower hepatic elasticity, while the grade of steatosis did not increase with rising BRI. A report by Iranian authors evaluated association between BRI and NAFLD in adult patients. In a group of patients with NAFLD the values of BRI were significantly higher than in a group of healthy subjects without NAFLD. Their results indicate, that higher BRI values are significantly associated with NAFLD in adults [12]. Similar studies aimed at paediatric populations are not currently available.
In clinical practice, transabdominal ultrasonography is the most frequently used imaging method in screening for NAFLD. Advantages of this method include non-invasiveness, wide availability, affordability and lack of radiation exposure [13]. However, it is not possible to reliably diagnose mild hepatic steatosis with the use of classical transabdominal ultrasonography, the method also does not allow differentiating between simple steatosis, non-alcohol induced steatohepatitis and hepatic fibrosis [14]. Hepatic steatosis is currently quantifiable using special software, and the grade of adipose infiltration of liver parenchyma can be determined using hepatorenal index values (HRI). Higher HRI values indicate a more severe hepatic steatosis. In a study by American and Italian authors, Shannon et al [15], liver steatosis was quantified via ultrasonographic imaging in paediatric patients with NAFLD verified by biopsy. During imaging, the examiner had no knowledge of clinical, laboratory, and histology data of the patients. Steatosis was evaluated using ultrasonographic scoring on a scale of 0 to 3. The results point to a significant correlation of ultrasonographic steatosis score with mild to severe grade of liver steatosis determined by histologic findings [15]. A team of Brazilian authors evaluated 42 adult patients with NAFLD confirmed by ultrasonography. These patients underwent liver biopsy with histologic examination and ultrasonographic imaging to determine their HRI values. The control group consisted of 40 healthy volunteers without steatosis and without any risk factors for NAFLD; the average HRI in this group was 1.09 ±0.13. In patients with NAFLD their HRI values were significantly correlated with the presence of steatosis confirmed by histology. HRI value of ≥1.24 was set as a cut-off for diagnosing steatosis with 92.7% sensitivity and 92.5% specificity [16]. These HRI values, same as HRI values presented in the study by Webb et al [5], are set for adult populations, therefore their use in paediatric medicine is questionable. HRI values in our cohort of 104 healthy paediatric subjects with normal BMI were however comparable with control group values presented by Borges et al [16]. No influence of age or sex of the patients in our control group on changes in HRI was observed. HRI values in our paediatric population were significantly higher in comparison with HRI of healthy term newborns. Lower values of HRI measured in the newborn cohort might be associated with elevated echogenicity of neonatal kidneys. It is apparent that a natural elevation of HRI occurs between neonatal and adolescent period. To determine the exact time when this switch happens, further research using larger cohorts of paediatric patients in various stages of childhood is needed. Evaluating whether breastfeeding and maternal risk factors have any influence on HRI values could also be beneficial. Adolescents with NAFLD with an anamnesis of being breastfed for at least 6 months had more favorable metabolic profiles compared to adolescent patients who were breastfed for less than 6 months. Supplementation with formula before 6th month of life was associated with higher prevalence of NAFLD and also with an increase in severity of the disease based on ultrasonographic findings [17]. Maternal obesity is associated with shorter breastfeeding period and subsequent early introduction of complementary milk formula and additional foods and eventually with unhealthy food preferences, which contributes to onset of childhood obesity [18]. Changes in tissue elasticity are caused by an onset of various pathological states. NAFLD can gradually progress from simple steatosis to non-alcohol induced steatohepatitis all the way to liver fibrosis or cirrhosis with changes of liver stiffness, which can be diagnosed by elastography. In our study, we evaluated changes in liver elasticity in individual patient groups using real-time elastography and compared them afterwards. In the neonatal group, we found statistically significant increase in liver tissue elasticity in comparison with the group of paediatric individuals with normal BMI. No influence of sex and age on changes in liver elasticity was observed the control paediatric group. In the general group of all obese individuals, we noted significantly lower levels of liver tissue elasticity compared to the control group. Liver elasticity in these patients decreased with increasing BMI. In addition, patients with obesity displayed a decrease in liver elasticity with increasing waist circumference compared to the control group of healthy children with normal weight. Using transient elastography, Italian authors Ferraioli et al [19] noted significantly higher levels of liver stiffness in children with liver steatosis in comparison with healthy children, the difference counting for approximately 0.5 Pa. This difference was however not described as statistically significant, because the lifer stiffness values were still within their normal range. The authors emphasis that liver steatosis is not always a benign condition, and should therefore be caught and adequately managed in its early stages [19]. Similar conclusions were reached by American authors from Phoenix in their study, which confirmed statistically significant differences in liver stiffness between children with obesity and children with normal body weight using ultrasound shear-wave elastography. They also noted increased liver stiffness in paediatric patients whose livers showed hyperechogenicity in conventional ultrasound examinations evocative of NAFLD. Ultrasound shear-wave elastography presents a reliable screening tool in quantification of liver stiffness and could be potentially helpful in early diagnosis and management of liver disease such as NAFLD and NASH in paediatric patients with obesity [20]. A group of authors from Osaka also concludes that transient elastography works as a non-invasive and effective method of screening for steatosis and liver fibrosis in obese paediatric patients [21]. Our results indicate that real-time elastography can detect shifts in liver tissue elasticity even before the onset of morphological alterations. Besides early detection of these changes, we are also able to evaluate progression of the disease and effectiveness of therapeutic intervention in real time, which improves management control. Real-time elastometry with the use of ultrasonography to evaluate tissue elasticity is a sensitive method in diagnosing early changes in liver elasticity in obese paediatric patients at risk for NAFLD.
Besides ultrasonography parameters, we also evaluated laboratory findings that might potentially be associated with liver damage in our cohort of all children with obesity. In the group of obese adolescent patients elevated HRI values were positively correlated with levels of hepatic enzymes ALT, AST and ALP, and also with markers of lipid metabolism such as TAGs, LDL and total serum cholesterol. Authors Fishbein et al (2003) present a finding that abnormalities in liver enzymes in paediatric patients with liver steatosis are present only in advanced stages of the disease. In patients with normal ALT levels however, a whole spectrum of NAFLD-associated disorders can be present [22]. High serum concentrations of GGT represent an elevated risk for advanced fibrosis in patients with NAFLD [23]. We did not observe an association between GGT and HRI/LFI in our group of obese pediatric patients. Thus, using ultrasound, we detected in this group a mild stage of liver fibrosis. Hypertriacylglycerolemia is another biochemical marker often found in obese children with NAFLD [24]. This type of dyslipidemia with lowered serum HDL levels typical in paediatric patients with obesity and is probably associated with insuline resistance (IR) [25]. In the cohort of all patients with obesity, we detected a decrease in serum D vitamin levels, the average vitamin D concentration settling within the range of hypovitaminosis [26]. These values were measured during winter time, when serum vitamin D is naturally low, therefore it would be beneficial to carry out a control measurement of vitamin D levels along with a control ultrasonography exam during the summer. Black et al (2014) researched a relationship between NAFLD and serum vitamin D levels in adolescent patients. Their results showed that the majority of patients with NAFLD had insufficient or deficient serum concentrations of vitamin D. Lower serum vitamin D levels were significantly correlated with an increase in NAFLD cases, independent of adiposity of IR [27].
Conclusion
The results of our study confirmed that obesity is justifiably considered to be the most important risk factor for the onset of NAFLD. Like BMI, the new obesity index – BRI – has no effect in predicting the stage of hepatic steatosis in paediatric populations. Identifying obese patients at risk and adequately managing the prevention of NAFLD and other diseases significantly associated with obesity is an important role of primary physicians. Obese pubescent and adolescent children with high values of waist circumference and elevated serum ALT levels regardless of gender should undergo ultrasound screening with establishing their hepatorenal index, as they are at significant risk for NAFLD. Based on hepatorenal index (HRI) values, we can precisely determine the stage of adipose infiltration of liver tissue in paediatric patients with obesity. Hepatic tissue elasticity decreases with increasing BMI and waist circumference in patients with obesity. Real-time elastography can detect qualitative changes in liver tissue even before they become apparent in ultrasound imaging. Ultrasonographic methods of determining HRI values and hepatic elasticity are applicable in diagnosing early stages of liver damage in obese pubescent and adolescent patients at risk for NAFLD.
ORCID authors
M. Pršo ORCID 0000-0003-0183-8158,
Z. Havlíčeková ORCID 0000-0001-5336-9942,
P. Bánovčin ORCID 0000-0001-6694-9364,
Ľ. Skladaný ORCID 0000-0001-5171-3623,
P. Bánovčin ORCID 0000-0003-0486-0123.
Submitted/Doručené: 12. 3. 2023
Accepted/Prijaté: 18. 3. 2023
Marek Kozár, MD, PhD.
Clinic of Neonatology
Jessenius Faculty of Medicine in Martin
Comenius University in Bratislava
University Hospital Martin
Kollárova 2
036 59 Martin
1marekkozar1@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Bodzsar EB, Zsakai A. Recent trends in childhood obesity and overweight in the transition countries of Eastern and Central Europe. Ann Hum Biol 2014; 41(3): 263–270. doi: 10.3109/03014460.2013.856473.
2. WHO. Obesity and overweight. 2021 [online]. Dostupné z: http: //who.int/mediacentre/factsheets/fs311/en/.
3. Cole TJ, Bellizzi MC, Flegal KM et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320(7244): 1240–1243. doi: 10.1136/bmj.320.7244.1240.
4. Thomas DM, Bredlau C, Bosy-Westphal A et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity (Silver Spring) 2013; 21(11): 2264–2271. doi: 10.1002/ oby.20408.
5. Webb M, Yeshua H, Zelber-Sagi S et al. Diag- nostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol 2009; 192(4): 909–914. doi: 10.2214/AJR.07.4016.
6. Kohli R, Sunduram S, Mouzaki M et al. Pediatric nonalcoholic fatty liver disease: a report from the expert committee on nonalcoholic fatty liver disease (ECON). J Pediatr 2016; 172: 9–13. doi: 10.1016/j.jpeds.2015.12.016.
7. Akcam M, Boyaci A, Pirgon O et al. Importance of the liver ultrasound scores in pubertal obese children with nonalcoholic fatty liver disease. Clin Imaging 2013; 37(3): 504–508. doi: 10.1016/j.clinimag.2012.07.011.
8. Hamaguchi M, Kojima T, Itoh Y et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102(12): 2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x.
9. Duarte MA, Silva GA. Hepatic steatosis in obese children and adolescents. J Pediatr 2011; 87(2): 150–156. doi: 10.2223/JPED.2065.
10. Vajro P, Lenta S, Socha P et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2012; 54(5): 700–713. doi: 10.1097/MPG.0b013e318252a13f.
11. Manco M, Bedogni G, Marcellini M et al. Waist circumference correlates with liver fibrosis in children with non-alcoholic steatohepatitis. Gut 2008; 57(9): 1283–1287. doi: 10.1136/gut. 2007.142919.
12. Motamed N, Rabiee B, Hemasi GR et al. Body Roundness Index and Waist-to-Height Ratio are Strongly Associated with Non-Alcoholic Fatty Liver Disease: A Population-Based Study. Hepat Mon 2016; 16(9): e39575. doi: 10.5812/hepatmon.39575.
13. Michnová Z, Szépeová R, Havlíčeková Z et al. Prevalencia hepatopatie u adolescentov s diabetes mellitus 1. typu. Pediatria (Bratisl.) 2017; 12(2): 53.
14. Saadeh S, Younossi ZM, Remer EM et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123(3): 745–750. doi: 10.1053/gast.2002.35354.
15. Shannon A, Alkhouri N, Carter-Kent C et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr 2011; 53(2): 190–195. doi: 10.1097/MPG.0b013e31821b4b61.
16. Borges VF, Diniz AL, Cotrim HP et al. Sonographic hepatorenal ratio: a noninvasive method to diagnose nonalcoholic steatosis. J Clin Ultrasound 2013; 41(1): 18–25. doi: 10.1002/jcu.21994.
17. Ayonrinde OT, Oddy WH, Adams LA et al. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol 2017; 67(3): 568–576. doi: 10.1016/j.jhep.2017.03.029.
18. Thompson AL. Intergenerational impact of maternal obesity and postnatal feeding practices on pediatric obesity. Nutr Rev 2013; 71(1): S55–S61. doi: 10.1111/nure.12054.
19. Ferraioli G, Calcaterra V, Lissandrin R et al. Noninvasive assessment of liver steatosis in children: the clinical value of controlled attenuation parameter. BMC Gastroenterol 2017; 17(1): 61. doi: 10.1186/s12876-017-0617-6.
20. Bailey SS, Youssfi M, Patel M et al. Shear-wave ultrasound elastography of the liver in normal-weight and obese children. Acta Radiologica 2017; 58(12): 1511–1518. doi: 10.1177/0284185117695668.
21. Cho Y, Tokuhara D, Morikawa H et al. Transient Elastography-Based Liver Profiles in a Hospital-Based Pediatric Population in Japan. PLoS One 2015; 10(9): e0137239. doi: 10.1371/journal.pone.0137239.
22. Fishbein MH, Miner M, Mogren C et al. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr 2003; 36(1): 54–61. doi: 10.1097/00005176-200301000-00012.
23. Havlíčeková Z, Szépeová R, Zubríková L et al. Význam črevnej mikroflóry v etiopatogenéze nealkoholovej steatózy pečene. Čes-slov Pediat 2015; 70(1): 11–16.
24. Schwimmer JB, Deutsch R, Kahen T et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118(4): 1388–1393. doi: 10.1542/peds.2006-1212.
25. Baker L, Farpour-Lambert JN, Nowicka P et al. Vyšetrenie dieťaťa s nadváhou/obezitou praktické rady pre primárnu lekársku starostlivosť: odporúčania od childhood obesity task force európskejobezitologickej spoločnosti (AESO). Pediatria (Bratisl.) 2012; 7(5): 229–234.
26. Račanská E. Vitamín D – hormón, ktorý nám chýba. Prakt. lekárn 2014; 4(2–3): 53–55.
27. Black LJ, Jacoby P, She Ping-Delfos WC et al. Low serum 25-hydroxyvitamin D concentracions associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol 2014; 29(6): 1215–1222. doi: 10.1111/jgh.12541.