Endoscopic findings in cirrhotic children candidates for liver transplantation
Seyed Mohsen Dehghani1, Maryam Abbasi2, Maryam Ataollahi1, Masoud Tahani3, Fateme Parooie3, Iraj Shahramian3
+ Affiliation
Summary
Background: Liver cirrhosis is an end-stage liver failure that can develop as a result of acute or chronic liver disease. Patients are at risk of fatal complications such as portal hypertension and bleeding from esophageal varices. Methods: This cross-sectional study examined the endoscopic findings in child patients <18 years with cirrhosis qualifying as candidates for liver transplantation. Our subjects were children admitted from 2012 to 2017 to the Shiraz Organ Transplantation Center of Nemazee Hospital, Shiraz University of Medical Sciences. The data was collected using a researcher-made questionnaire. Results: We studied 199 patients (49.2% boys) with liver cirrhosis admitted for upper gastrointestinal endoscopy. Their average age was 6.2 ±4.7 years old. The most common clinical sign present at admission was icterus (58.8%). The mean values of the children’s Child-Pugh and PELD/MELD scores were 8.53 ±2.34 and 14.85 ±14.93, respectively. Of 199 children examined, 145 total (72.8%) suffered from esophageal varices, further divided by severity into grades 1, 2, 3, and 4 – 39 (19.7%), 53 (26.8%), 42 (21.2%), 11 (5.6%), respectively. Further symptoms, such as portal hypertensive gastropathy (PHG), gastric erythema, fundal varices, gastric erosion, and gastric ulcer were found in 31.1%, 15.1%, 6.5%, 5.5%, and 2% of the patients, respectively. No associations were observed between esophageal or gastric varices and Child-Pugh (P = 0.076), PELD/MELD score (P = 0.607), clinical symptoms, laboratory parameters, or underlying diseases. Conclusion: Our patients’ main cause of liver cirrhosis was biliary atresia, and the most common presenting sign in endoscopy was esophageal varices.
Keywords
biliary atresia, esophageal varices, jaundice, endoscopic treatment
Introduction
The natural course of liver cirrhosis consists of two stages, compensated and decompensated [1,2]. Disease progression from the compensated phase to the decompensated one is characterized by symptoms such as bleeding from esophageal varices, ascites, encephalopathy, and icterus. The survival of patients in these two stages of cirrhosis is dramatically different, with the median survival rate of patients in the compensated stage being 12 years, whereas patients in the decompensated stage only had 2 years on average [1,3].
At the time of diagnosis of cirrhosis, esophageal varices are observed in 30–40% of patients with compensated cirrhosis and in 60% of patients with ascites [4]. Children with decompensated cirrhosis are at a higher risk of upper gastrointestinal bleedings from esophageal and gastric varices while awaiting liver transplantation [5]. In the United States, 30% of patients with compensated cirrhosis will suffer bleeding from esophageal varices [6], which also constitutes the most common cause of death for patients with cirrhosis [7]. The mortality rate due to one episode of bleeding from esophageal varices reaches 15 to 20% [8]. Overall, around half of the patients with liver cirrhosis develop varices due to portal hypertension, and 40% of these patients will experience bleeding from varices [9,10].
The upper gastrointestinal (GI) endoscopy is the gold standard method of diagnosis of varices [11]. Endoscopy is the best tool for assessing the presence of esophageal, gastric, and sometimes duodenal varices [12]. The aim of our study was to assess upper GI endoscopy findings in child candidates for liver transplantation due to liver cirrhosis in a referral center in Shiraz, Iran.
Patients and methods
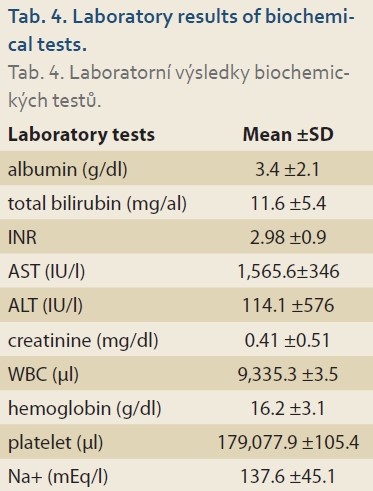
This was a cross-sectional study on children <18 years old with liver cirrhosis enlisted for liver transplantation in the Organ Transplantation Center, Nemazee Hospital, Shiraz University of Medical Sciences, from 2012 to 2017. The data was collected using a researcher--made questionnaire, which included questions regarding the patient‘s age, gender, the presence or absence of esophageal varices and other endoscopy findings, symptoms at the time of admission, MELD/PELD and Child-Pugh scores, the results of the biochemical laboratory tests including serum albumin, bilirubin, AST, ALT, creatinine, INR, the counts of white blood cells and platelets, as well as hemoglobin. A total number of 199 children participated in this study. Statistical analyses were performed in SPSS version 25 software using descriptive and analytical tests.
Results
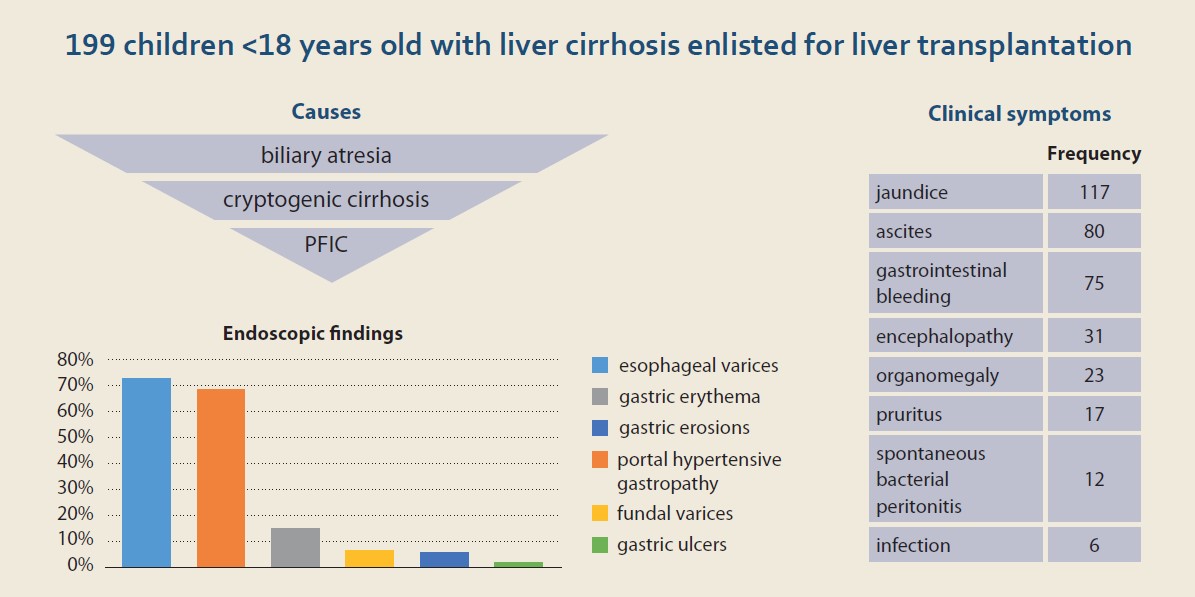
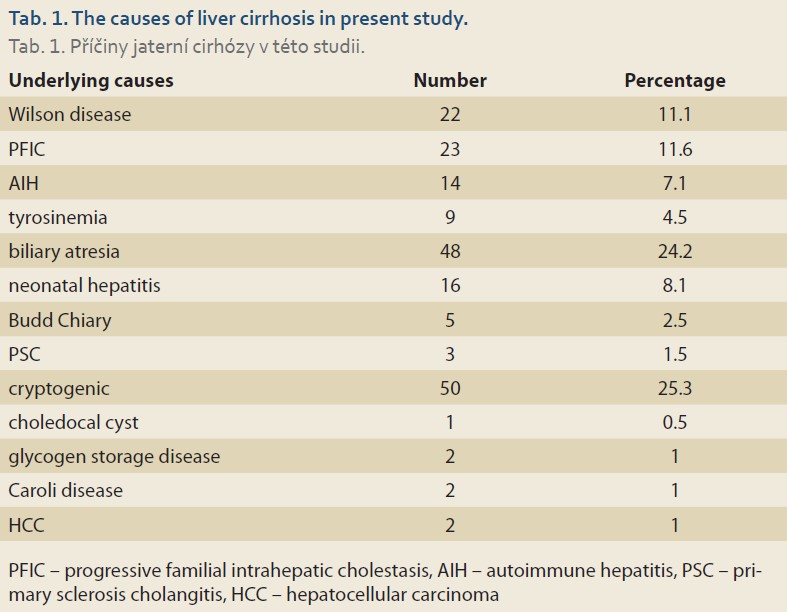
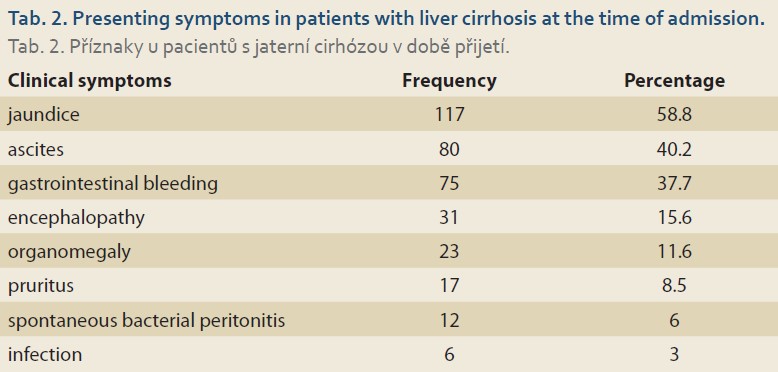
Of the 199 children examined, 98 (49.2%) were boys and 101 (50.8%) girls. The mean age of the patients was 6.2 ±4.7 years old. Biliary atresia was the most common underlying cause of our patients’ liver cirrhosis (N = 50; 25.3%), followed by cryptogenic cirrhosis (N = 48; 24.2%), and progressive familial intrahepatic cholestasis (PFIC) (N = 23; 11.6%) (Tab. 1). The most common presenting symptoms were icterus (N = 117; 58.8%), ascites (N = 80; 40.2%), and gastrointestinal bleeding (N = 75; 37.7%) (Tab. 2).
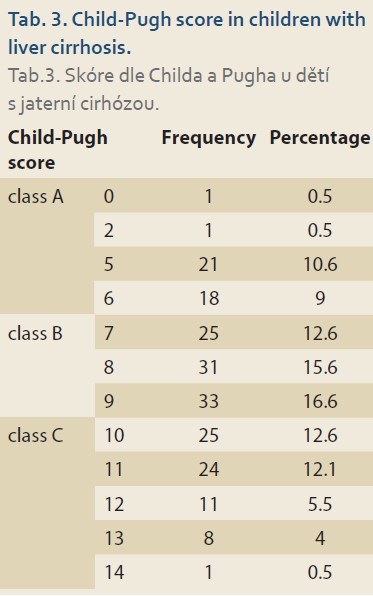
The mean Child-Pugh score was 8.53 ±2.34. Most of the patients belonged to the class B (N = 89; 44.7%) (Tab. 3). The average MELD/PELD score calculated was 14.85 ±14.93 (median = 11, minimum = 1, and maximum = 47). The mean levels of laboratory biochemical tests are shown in Tab. 4.
Endoscopic findings
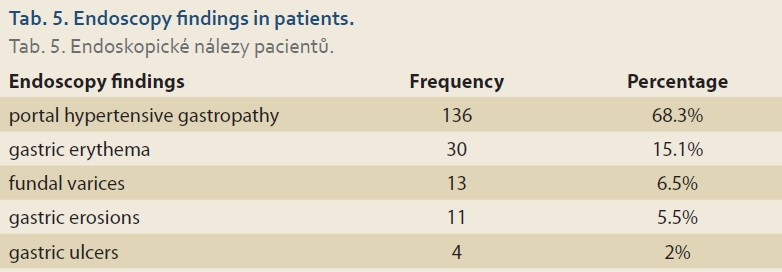
Esophageal varices were the most common finding. Out of 199 patients who have undergone upper gastrointestinal endoscopy, 39 (19.7%), 53 (26.8%), 42 (21.2%), and 11 (5.6%) had grade 1, 2, 3, and 4 esophageal varices, respectively. The next most prevalent finding was portal hypertensive gastropathy (68.3%), defined as reticulated or mosaic gastric mucosa. The third most prevalent finding was gastric erythema (15.1%), followed by fundal varices (6.5%) and gastric erosions (5.5%). In 4 (2%) of the patients, the endoscopic evaluation revealed gastric ulcers (Tab. 5).
There were no associations between esophageal or gastric varices and Child-Pugh (P = 0.076), PELD/MELD score (P = 0.607), clinical symptoms, laboratory parameters, or underlying diseases.
Discussion
Of the 199 cirrhotic children who had upper gastrointestinal endoscopy, 145 (72.8%) had esophageal varices. Other symptoms, such as PHG, gastric erythema, fundal varices, gastric erosion, and gastric ulcer were found in 31.1%, 15.1%, 6.5%, 5.5%, and 2% of the patients, respectively. The most common underlying disease was biliary atresia and the most common clinical symptom was icterus. The mean age of the patients in this study was 6.2 years old. The average scores of Child-Pugh and MELD/PELD in this study were 8.53 and 14.85, respectively.
In this study, the number of boys and girls with liver cirrhosis was approximately equal. This was similar to the results of a study by Chaabouni et al in Tunisia [13]. However, this finding was opposed to the results of Dehghani et al in Shiraz, in which the number of boys was significantly higher than that of girls [14,15]. On the other hand, Ng et al, in a study in Singapore, reported the number of girls with liver cirrhosis to be significantly higher than the number of boys [16]. We identified biliary atresia as the main cause of liver cirrhosis. This result was different from those of Chaabouni et al in Tunisia [13], Ng et al in Singapore [16], Bonnet et al in Belgium [17], and Dehghani et al in Shiraz [14]. Tumgor et al, in a study in Turkey, reported metabolic diseases as the most common underlying causes of liver cirrhosis in children, followed by biliary atresia [18]. However, Dehghani et al stated that the most frequent underlying cause of liver cirrhosis was Wilson disease and then biliary atresia [15]. Overall, it seems that biliary atresia comprises one of the most common causes of liver cirrhosis in children.
The most common clinical symptom in our patients was icterus, which was in line with studies of Chaabouni et al in Tunisia [13] and Dehghani et al in Shiraz [14,15]. Most of our patients belonged to class B of the Child-Pugh scoring system, which was in accordance with the results of Dehghani et al in Shiraz [15].
Esophageal varices are particularly common in patients with liver cirrhosis. Here, 63.2% of the patients showed esophageal varices in upper GI endoscopy examination. This high rate is consistent with most prior studies, which have shown rates of 39.5% [19], 49.9% [20], and 68.3% [21]. The development of esophageal varices has been associated with various risk factors; including Child-Pugh class, platelet count, liver fibrosis severity [22], spleen stiffness [23,24], liver to spleen ratio [25], advanced age, liver disease, and increased splenic and portal vein diameters [19]. Esophageal varices should be properly managed as they are often associated with bleeding episodes, experienced by up to 45% of the patients [26]. Non-invasive predictors of esophageal varices and assessment of the risk of bleeding from them in particular can help avoid invasive endoscopy procedures [27]. For that purpose, the Baveno VI criteria (i.e., platelet count > 150 × 109/L and liver stiffness measurement <20 kPa) have been suggested as a reliable non-invasive indicator for prediction of esophageal varices [28]. A new score (the Liaoning score), combining various laboratory and clinical parameters to predict esophageal varices in cirrhotic patients, was suggested by a Chinese team [29].
We detected PHG in 31.1% of the patients. This is a common diagnosis in cirrhotic patients and it can lead to bleeding [30,31] or even iron-deficiency anemia [30]. In endoscopy examination, which is the gold standard of diagnostic procedures for PHG, gastric mucosa appears as a mosaic-like image that may or may not be accompanied by red spots [32]. A study by Singh et al shows 30 out of 80 children (37.5%) with extrahepatic portal vein obstruction (EHPVO) suffering from portal hypertensive gastropathy (PHG) [33]. In another study of 50 children suffering from end-stage liver disease (ESLD), PHG was detected in 46% of them [34]. Min et al in their study on adults with chronic liver disease identified PHG in 21.1% [35]. In an examination of patients with chronic hepatitis C infection, a half developed PHG during the four years following the diagnosis, while 26% of patients with PHG already present progressed towards a more severe condition [36].
Simbrunner et al found an association between PHG and male gender, the Child-Pugh score, and hemoglobin level, but not the MELD score [30]. Furthermore, the Helicobacter pylori infection was noted to double the risk of PHG (OR = 2.1; 95% CI 1–4.3; P = 0.034) [37]. In another study on patients with chronic liver diseases, mainly (70%) due to secondary to viral hepatitis B and C infections, platelet count and serum albumin were associated with developing PHG [35]. Another study on patients with chronic hepatitis C infection found a correlation between elevated alkaline phosphatase, a higher AST/ALT ratio, low albumin level, diabetes, and Caucasian ethnicity and either new development of PHG or its further progression [36].
We found gastric erythema in 15.1% of our patients, fundal varices in 6.5%, gastric erosions in 5.5%, and gastric ulcers in 2% of them. Kunihara et al, in their investigation of 50 liver cirrhosis adult patients, reported gastric erythema in 28%, erosions in 18%, and villous edema in 16% [38]. In another study on 162 adults with liver cirrhosis, fundal varices were seen in 7.4% of the patients [39]. De Faria et al studied 50 adults with chronic liver disease (mainly, 66%, due to viral hepatitis) and reported gastric varices in only 2 (4%) patients [40]. On the other hand, among 205 adults with liver cirrhosis due to hepatitis C infection, gastric varices were reported in 30 (14.6%) patients [41]. Of 80 children with EHPVO who underwent endoscopy, gastric varices were seen in 3.8% of them [33]. Although endoscopy is the gold standard method for detecting varices in the gastrointestinal tract, continuous efforts are dedicated to developing new non-invasive indicators for this phenomenon [42]. Gastric varices, along with esophageal varices, are important risk factors for bleeding episodes and mortality in patients with liver cirrhosis and therefore need special attention [43]. Overall, 10 to 30% of patients with liver cirrhosis were reported to have developed gastric varices that can be associated with up to 45% mortality [44].
Conclusion
In conclusion, biliary atresia was the main cause of liver cirrhosis in our subjects, children <18 years old. Their most common clinical sign was icterus. Upper GI endoscopy found esophageal varices as the main symptom for most of the patients. As bleeding from varices is the most common associated cause of mortality, it is advisable to routinely check the patients for evidence of esophageal and gastric varices.
ORCID authors
S. M. Dehghani ORCID 0000-0001-5930-0110,
M. Abbasi ORCID 0000-0002-9905-0395,
M. Ataollahi ORCID 0000-0003-1876-5266,
M. Tahani ORCID 0000-0002-0213-9087,
F. Parooie ORCID 0000-0002-2367-3780,
I. Shahramian ORCID 0000-0002-3760-7717.
Submitted/Doručeno: 7. 6. 2022
Accepted/Přijato: 30. 10. 2022
Prof. Iraj Shahramian, MD
Pediatric Gastroenterology and Hepatology Research Center
Zabol University of Medical Sciences
Shahid Rajaei Street 9861615881 Zabol
Iran
irajshahramian@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. D‘Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44(1): 217–231. doi: 10.1016/j.jhep.2005.10.013.
2. Garcia‐Tsao G, Friedman S, Iredale J et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010; 51(4): 1445–1449. doi: 10.1002/hep.23478.
3. Ginés P, Quintero E, Arroyo V et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987; 7(1): 122–128. doi: 10.1002/hep.1840070124.
4. D’Amico G. Esophageal varices: from appearance to rupture; natural history and prognostic indicators. Portal hypertension in the 21st Century. Springer 2004; 147–154. doi: 10.1007/ 978-94-007-1042-9_17.
5. Owensby S, Taylor K, Wilkins T. Diagnosis and management of upper gastrointestinal bleeding in children. J Am Board Fam Med 2015; 28(1): 134–145. doi: 10.3122/jabfm.2015.01.140 153.
6. D‘Amico G, Garcia‐Tsao G, Cales P et al. Session 2 – Diagnosis of Portal Hypertension: How and When. In: Portal Hypertension III: Pro- ceedings of the Third Baverno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies; 2001: Wiley Online Library. doi: 10.1002/97804707601 54.ch4.
7. Gado AS, Ebeid BA, Abdelmohsen AM et al. Clinical outcome of acute upper gastrointestinal hemorrhage among patients admitted to a government hospital in Egypt. Saudi J Gastroenterol 2012; 18(1): 34–39. doi: 10.4103/13 19-3767.91737.
8. Carbonell N, Pauwels A, Serfaty L et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004; 40(3): 652–659. doi: 10.1002/hep.20339.
9. Toubia N, Sanyal AJ. Portal hypertension and variceal hemorrhage. Med Clin North Am 2008; 92(3): 551–574, viii. doi: 10.1016/ j.mcna.2007.12.003.
10. Merli M, Nicolini G, Angeloni S et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol 2003; 38(3): 266–272. doi: 10.1016/s0168-8278(02)00420-8.
11. Garcia‐Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding – Unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single‐topic conference. Hepatology 2008; 47(5): 1764–1772. doi: 10.1002/hep.22273.
12. Pinto RB, Schneider AC, da Silveira TR. Cirrhosis in children and adolescents: An overview. World J Hepatol 2015; 7(3): 392–405. doi: 10.4254/wjh.v7.i3.392.
13. Chaabouni M, Bahloul S, Ben Romdhane W et al. Epidemiological, etiological and evolutionary aspects of children cirrhosis in a developing country: experience of the pediatric department of SFAX University hospital, Tunisia. Tunis Med 2007; 85(9): 738–743.
14. Dehghani SM, Shahramian I, Bazi A et al. Evaluation of Underlying Liver Disease and Its Severity in Children Referred for Liver Transplant: a Single-Center Report From Nemazee Hospital of Shiraz. Exp Clin Transplant 2020; 18(7): 803–807. doi: 10.6002/ect.2018.0047.
15. Dehghani SM, Imanieh MH, Haghighat M et al. Etiology and complications of liver cirrhosis in children: report of a single center from southern iran. Middle East J Dig Dis: 2013; 5(1): 41–46.
16. Ng NB, Karthik SV, Aw MM et al. Endoscopic Evaluation in Children With End-Stage Liver Disease-Associated Portal Hypertension Awaiting Liver Transplant. J Pediatr Gastroenterol Nutr 2016; 63(3): 365–369. doi: 10.1097/ MPG.0000000000001160.
17. Bonnet N, Paul J, Helleputte T et al. Novel insights into the assessment of risk of upper gastrointestinal bleeding in decompensated cirrhotic children. Pediatr Transplant 2019; 23(4): e13390. doi: 10.1111/petr.13390.
18. Tumgor G, Arikan C, Yuksekkaya HA et al. Childhood cirrhosis, hepatopulmonary syndrome and liver transplantation. Pediatr Transplant 2008; 12(3): 353–357. doi: 10.1111/j.13 99-3046. 2007.00807.x.
19. Gunda DW, Kilonzo SB, Mamballah Z et al. The magnitude and correlates of esophageal Varices among newly diagnosed cirrhotic patients undergoing screening fibre optic endoscope before incident bleeding in North-Western Tanzania; a cross-sectional study. BMC Gastroenterol 2019; 19(1): 203. doi: 10.1186/s12876-019-1123-9.
20. Saleh ZM, Solano QP, Louissaint J et al. The incidence and outcome of postoperative hepatic encephalopathy in patients with cirrhosis. United European Gastroenterol 2021; 9(6): 672–680. doi: 10.1002/ueg2.12104.
21. Fujiyama S, Akuta N, Sezaki H et al. Mortality rates and risk factors in 1412 Japanese patients with decompensated hepatitis C virus-related cirrhosis: a retrospective long-term cohort study. BMC Gastroenterol 2021; 21(1): 189. doi: 10.1186/s12876-021-01770-0.
22. Xu SH, Wu F, Guo LH et al. Liver fibrosis index-based nomograms for identifying esophageal varices in patients with chronic hepatitis B related cirrhosis. World J Gastroenterol 2020; 26(45): 7204–7221. doi: 10.3748/wjg.v26.i45. 7204.
23. Xu X, Jin Y, Lin Y et al. Multimodal Ultrasound Model Based on the Left Gastric Vein in B-Viral Cirrhosis: Noninvasive Prediction of Esophageal Varices. Clin Transl gastroenterol 2020; 11(1): e00262. doi: 10.14309/ctg.0000000000000262.
24. Fierbinteanu-Braticevici C, Tribus L, Peagu R et al. Spleen Stiffness as Predictor of Esophageal Varices in Cirrhosis of Different Etiologies. Sci Rep 2019; 9(1): 16190. doi: 10.1038/s4159 8-019-52407-y.
25. Yang LB, Xu JY, Tantai XX et al. Non-invasive prediction model for high-risk esophageal varices in the Chinese population. World J Gastroenterol 2020; 26(21): 2839–2851. doi: 10.3748/wjg.v26.i21.2839.
26. Ekmen N, Cifci S. Evaluation of the Relationship Between Pulmonary Artery Hypertension and Esophageal Varices Bleeding in Transplantation Candidates. Cureus 2021; 13(2): e13355. doi: 10.7759/cureus.13355.
27. Takehara T, Sakamori R. Remaining challenges for the noninvasive diagnosis of esophageal varices in liver cirrhosis. Esophagus 2020; 17(1): 19–24. doi: 10.1007/s10388-019-00699-4.
28. Zhou H, Long J, Hu H et al. Liver stiffness and serum markers for excluding high-risk varices in patients who do not meet Baveno VI criteria. World J Gastroenterol 2019; 25(35): 5323–5333. doi: 10.3748/wjg.v25.i35.5323.
29. Qi X, Li Y, Wang R et al. Liaoning Score for Prediction of Esophageal Varices in Cirrhotic Patients Who Had Never Undergone Endoscopy: A Multicenter Cross-Sectional Study in Liaoning Province, China. Adv Ther 2019; 36(8): 2167–2178. doi: 10.1007/s12325-019-00967-w.
30. Simbrunner B, Beer A, Wöran K et al. Portal hypertensive gastropathy is associated with iron deficiency anemia. Wien Klin Wochenschr 2020; 132(1–2): 1–11. doi: 10.1007/s00 508-019-01593-w.
31. Purbey BK, Gurung RB, Panday R et al. The Etiology of Upper Gastrointestinal Bleeding in Patients with Liver Cirrhosis in Dhulikhel Hospital. Kathmandu Univ Med J (KUMJ) 2017; 15(60): 292–295.
32. Mandhwani R, Hanif FM, Ul Haque MM et al. Noninvasive Clinical Predictors of Portal Hypertensive Gastropathy in Patients with Liver Cirrhosis. J Transl Int Med 2017; 5(3): 169–173. doi: 10.1515/jtim-2017-0025.
33. Singh S, Bhamre R, Shetty N et al. Correlation of endoscopic findings with Doppler ultrasound in portal hypertension in children. Clin Exp Hepatol 2021; 7(2): 191–195. doi: 10.5114/ceh.2021.106509.
34. Ng NBH, Karthik SV, Aw MM et al. Endoscopic Evaluation in Children With End-Stage Liver Disease-Associated Portal Hypertension Awaiting Liver Transplant. J Pediatr Gastroenterol Nutr 2016; 63(3): 365–369. doi: 10.1097/MPG.0000000000001160.
35. Min YW, Bae SY, Gwak GY et al. A clinical predictor of varices and portal hypertensive gastropathy in patients with chronic liver disease. Clin Mol Hepatol 2012; 18(2): 178–184. doi: 10.3350/cmh.2012.18.2.178.
36. Fontana RJ, Sanyal AJ, Ghany MG et al. Development and progression of portal hypertensive gastropathy in patients with chronic hepatitis C. Am J Gastroenterol 2011; 106(5): 884–893. doi: 10.1038/ajg.2010.456.
37. Sathar SA, Kunnathuparambil SG, Sreesh S et al. Helicobacter pylori infection in patients with liver cirrhosis: prevalence and association with portal hypertensive gastropathy. Ann Gastroenterol 2014; 27(1): 48–52.
38. Kunihara S, Oka S, Tanaka S et al. Predictive Factors of Portal Hypertensive Enteropathy Exacerbation in Patients with Liver Cirrhosis: A Capsule Endoscopy Study Digestion 2018; 98(1): 33–40. doi: 10.1159/000486666.
39. Samiullah S, Memon MS, Memon HG et al. Secondary gastric varices in hepatic cirrhosis. J Coll Physicians Surg Pak 2011; 21(10): 593–596. doi: 10.2011/JCPSP.593596.
40. De Faria AA, Dias CAF, Dias Moetzsohn L et al. Feasibility of transnasal endoscopy in screening for esophageal and gastric varices in patients with chronic liver disease. Endosc Int Open 2017; 5(7): 646–651. doi: 10.1055/s-0043-107781.
41. Butt Z, Ali Shah SM, Afzal M et al. Frequency of different types of gastric varices in patients with cirrhosis due to chronic hepatitis C. J Pak Med Assoc 2016; 66(11): 1462–1465.
42. Petrisor A, Stanescu AMA, Papacocea IR et al. Non-invasive laboratory, imaging and elastography markers in predicting varices with high risk of bleeding in cirrhotic patients. Rom J Intern Med 2021; 59(2): 194–200. doi: 10.2478/rjim-2021-0001.
43. Lu Z, Sun X, Han J et al. Characteristics of peptic ulcer bleeding in cirrhotic patients with esophageal and gastric varices. Sci Rep 2020; 10(1): 20068. doi: 10.1038/s41598-020-76530-3.
44. Lesmana CRA, Kalista KF, Sandra S et al. Clinical significance of isolated gastric varices in liver cirrhotic patients: A single-referral-centre retrospective cohort study. JGH Open 2019; 4(3): 511–518. doi: 10.1002/jgh3.12292.