Diferenciální diagnostika rekurentní idiopatické akutní pankreatitidy
Jan Trna Orcid.org 1, Veronika Příbramská Orcid.org , Petr Dítě Orcid.org 2,3
+ Affiliation
Summary
SOUHRN
Trna J, Příbramská V, Dítě P. Diferenciální diagnostika rekurentní idiopatické akutní pankreatitidy
Diferenciální diagnostika a léčba pacientů s idiopatickou rekurentní akutní pankreatitidou představují celosvětový problém. Kvalita života postižených jedinců bývá významně ovlivněna a zanedbatelné nejsou ani kapacitní a finanční náklady, které musí zdravotnické systémy vynaložit. Diskuse o ideálním diagnosticko-terapeutickém postupu se vedou po desetiletí. S rozvojem a lepší dostupností nových zobrazovacích a laboratorních metod se u mnoha pacientů podaří stanovit přesnou diagnózu. U těch, u kterých se přesto nepodaří příčinu vypátrat, zůstavá nejvhodnější postup nejasný.
Tento přehledový článek podává výčet možných etiologických faktorů a detailní popis jednotlivých diagnostických nástrojů dostupných pro pankreatické afekce. V závěru shrnujeme diagnosticko-terapeutické postupy založené na literárních údajích i vlastních datech.
Klíčová slova: endosonografie - jaterní testy - mikrolitiáza - rekurentní akutní pankreatitida.
INTRODUCTION
Acute pancreatitis (AP) is a serious condition with considerable morbidity and mortality about 7% which may increase to 17-39% in patients with severe disease(1-3). The cause of AP is easy to diagnose in 70% to 90% with alcohol and gallstones being the most common(4,5). Metabolic disorders, drugs, anatomic abnormalities, viral infections, trauma, tumors and variety of other rare causes are implicated in about 10%(1,3,6,7). In remaining 10% to 20% the etiology remains unknown after standard investigation and patients are discharged with diagnose of idiopathic acute pancreatitis (IAP). There is a conflicting body of evidence in the world literature regarding recurrence rate of IAP. The recurrence rate varies within the studies widely between as low as 4% to as high as 60%(8,9). According to the most of the authors the "wait and see" strategy after the first episode of IAP with extensive evaluation delayed after second attack is widely accepted and fairly reasoned(3,10). Although this strategy should not be applied to persons of older age (over 40 years) because of higher risk of underlying malignancy(11). In the group of patients 40 to 60 years old investigated for IRAP the malignancy was found as an underlying cause in 21% and in patients older than 60 years in 25% compared to only 3% in group of patients younger than 40 years(12).
If the underlying etiology of recurring episodes remains enigmatic despite further investigation patients are labeled as having idiopathic recurrent acute pancreatitis (IRAP). The extent of the evaluation impacts the frequency with which an etiology is found and how often the diagnosis of IRAP is issued. If extended work-up fails in revealing the etiology the diagnosis of "true" IRAP can be assigned(3,13).
ETIOLOGICAL FACTORS
Early stage chronic pancreatitis (CP)
Early stage CP often presents itself with recurrent acute exacerbations without typical findings on imaging methods as CT or ERCP(7,14,15). For example according to study from New Deli 47% of patients initially diagnosed with IRAP were proved as having CP during follow-up(16). Endoscopic ultrasound (EUS) is a relatively new method which offers the possibility of diagnosing earlier stages of CP than CT, thus the results are partly conflicting. The direct pancreatic function testing is invasive and not commonly available in clinical practice(17). Sometimes only careful follow-up of patients with IRAP will lead to development of diagnostic criteria of CP on imaging methods.
Biliary microlithiasis, sludge, crystals
Several reports suggest that a large portion of patients diagnosed with IAP do in fact have biliary pancreatitis caused by microlithiasis or sludge which is undetectable by conventional imaging techniques(18,19). Smaller gallstones carry out a higher risk for causing AP than large ones supporting the possible role of microlithiasis(20-22). Microliths are prone to migrate into the common bile duct (CBD) and are likely to become impacted in the common channel thus triggering AP(19,23). The terms microlithiasis and sludge are quite often used interchangeably. Despite the differences, in most studies biliary microlithiasis is defined as presence of stones smaller than 3 mm in diameter, in absence of macroscopic stones(11,24). Biliary sludge on the other hand consists of suspension of crystals (cholesterol monohydrate crystals, calcium bilirubinate granules, calcium carbonate salts), mucin, glycoproteins and cellular debris(19,25).
Some reports found microlithiasis as a cause of IRAP in less than 10%(16,26, 27). Most have found the evidence of microlithiasis in about two thirds of patients(13,23,28). Most investigators believe that in absence of another identifiable risk factor the presence of microlithiasis in patient with AP is enough of evidence for causality(13). Some authors recommend routine search for microlithiasis presence(18,25) others question this practice(29).
The presence of elevated liver function tests (FLT) strongly suggests biliary etiology of AP. The only study evaluating the FLT levels in patients with IAP and comparing the results with MBE findings confirmed that serum increase of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) levels is suggestive of microlithiasis etiology of IAP especially if assessed early (within 24 hours) after the onset of abdominal pain(30). The results were comparable to results found in patients with biliary pancreatitis caused by macroscopic gallstones(30,31). However, sensitivity and specificity of these tests were significantly lower if assessed between 24 and 72 hours. These findings suggest that AP associated with microlithiasis may have a similar behavior to AP with unequivocal gallstones(30). In significant portion of biliary AP LFTs are however normal and therefore no biochemical parameter can currently be considered a reliable indicator of biliary etiology of AP(32).
Bile microscopy and EUS are other possible options for microlithiasis detection. However, these methods are not generally available and world literature data are insufficient and partly controversial. If microlithiasis and/or biliary sludge are proven or strongly suspected to be the causing factor of AP cholecystectomy is strongly recommended as treatment of choice(18, 25) with endoscopic sphincterectomy as an alternative for elderly of surgically unfit patients(13,25,33-35).
Bile crystals are proven to be rare in patients with manometrically proven Sphincter of Oddi dysfunction(36).
Sphincter of Oddi dysfunction (SOD)
Sphincter of Oddi dysfunction is a controversial cause of IAP. SOD is usually defined as a high basal biliary or pancreatic sphincter pressure of more than 40 mm Hg(37). Such findings are referred to be present in about 25% to 60% of patients with IAP(38-41) but also in patients with pain only and no prior pancreatitis(40). The pathogenesis is related either to passive obstruction caused by fibrosis and/ or inflammation or to active obstruction caused by sphincter muscle spasm. These two mechanisms are not mutually exclusive(40).
It is generally unclear if this condition is original etiology of AP attack or result of prior pancreatic inflammation but because of decrease in recurrent episodes after sphincter ablation a role of SOD is likely. However, the data come mostly from smaller retrospective studies. These patients are then commonly advised to undergo endoscopic or surgical treatment(41).
However, the main problems are unavailability and invasiveness of the sphincter of Oddi manometry (SOM). Moreover, there is a lack of data allowing us to establish normal values of sphincter of Oddi pressure. Sphincter of Oddi manometry should be therefore considered experimental method not routinely performed in clinical practice(42). Sphincter of Oddi dysfunction is believed to be relatively common cause of IRAP in post-cholecystectomy patients(23). Thus SOM should be reserved for patients with recurrent AP after CCX(43).
Pancreas divisum
Pancreas divisum is the most common congenital anomaly of the pancreatic duct, present in 5 to 14% of individuals(44). It arises from failure of fusion of the dorsal and ventral pancreatic ducts, causing the majority of pancreatic juice to flow through the minor papilla(45). Its role as etiological factor of recurrent AP is also controversial. Ductal hypertension as a result of obstructed pancreatic juice flow at the level of inadequately patent or stenosed minor papilla is presumed to be the triggering factor of recurrent attacks of AP(44). Therefore ERCP or MRCP should be part of evaluation of recurrent AP to exclude pancreas divisum(6). New data suggest that pancreas divisum can be also diagnosed by EUS(46). In case of proven duct dilation endoscopic or surgical sphincteroplasty of minor papilla may lead to resolution of IRAP in this subgroup of patients with pancreas divisum(47). Long-term outcome studies are however missing.
DIAGNOSTIC TOOLS
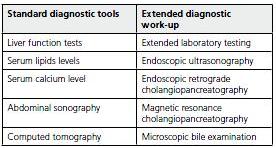
Function liver tests (FLT)
Elevation of function liver tests within 48 hours of admission is well documented to be a reliable evidence of biliary etiology of AP(48). The levels were observed to fall over the subsequent 48 hours(48). This transient increase in serum levels of FLT (especially aminotransferases) is being explained by necrosis and/or hepatocellular degeneration secondary to ampullary impaction of calculi during migration(49). According to metaanalysis from 1994 threefold elevation of ALT level was connected with sensitivity of 48% and specificity 96% and positive predictive value of 95%(31). ALT was proven to be the most clinically useful parameter among FLT in diagnosing gallstone pancreatitis, with AST being almost as useful(31). However, almost 15 to 20% of patients with biliary AP manifest with normal LFTs. The clinician should not exclude a biliary etiology solely on the basis of normal LFTs and a combination of diagnostic procedures is required for establishing the biliary etiology for AP(32).
Transabdominal ultrasonography
US is a non-invasive method with sensitivity ranging between 86% and 96% and specificity between 93% and 100% in detecting gallbladder stones(33,50-53), common bile duct dilation between 55% to 91%(54) and choledocholithiasis somewhere between 20% to 75%(11,55). False negative results can be caused by stones < 3 mm in diameter which are below US resolution. In case of stones located in gall bladder infundibulum the sensitivity drops to only 65%(33,56). Newer data suggest that US is capable of detection of the combination of mucus and crystals greater than 50 um but in very operator-dependent manner and with low sensitivity of about 50-60%(13,55). Repeated examination can improve the diagnostic yield.
Combination of US and FLT reached sensitivity of 81-98%, specificity 100%, positive and negative predictive value of 100% and 96% respectively(2,52) and therefore indicates gallstones correctly as a cause of AP in the majority of patients.
Contrast enhanced computed tomography (CT)
Contrast enhanced CT is more accurate in confirmation of the diagnosis of AP and assessment of its severity(57). CT demonstrates swelling of the gland, peripancreatic inflammation and later in the course of the disease possible necrosis and complications such as fluid collections(53).
Extended laboratory testing
Recurrent idiopathic acute pancreatitis can be sometimes caused by underlying genetic etiology. Mutations in the cationic trypsinogen gene (PRSS1) are responsible for hereditary chronic pancreatitis which in early form often presents as episodes of AP without clear etiology(58). Also mutations in cystic fibrosis transmembrane conductance regulator (CFTR) and pancreatic secretory trypsin inhibitor gene (SPINK1) have been identified with increased frequency in idiopathic acute pancreatitis patients(7,59).
An autoimmune etiology should be considered especially if patient has any other "rheumatologic" disease (e.g. Sjögren syndrome) and the IgG4 level is elevated(46). Blood testing of present antibodies together with typical finding of so called sausage- like pancreas on CT or other imaging methods can reveal autoimmune etiology which can be then treated with glucocorticoids(60).
Endoscopic ultrasonography (EUS)
EUS is a relatively new method which is considered to be superior to US and CT for imaging of pancreaticobilliary system(61). In a study evaluating 168 patients with IAP, an abnormality was detected by EUS in 80%, with 62% being biliary tract pathology such as stones, sludge or microlithiasis. Correct diagnosis was established usually during surgery and EUS reached 92% accuracy(62).
EUS seems to be the most sensitive procedure for the diagnosis of choledocholithiasis with both 90%(63,64) specificity and sensitivity over. It was proposed that EUS performed prior ERCP and/or CCX can identify patients with choledocholithiasis and thereby avoid unnecessary instrumental exploration of the duct(61,65). EUS should be considered in patients with negative US and biochemical profile indicating biliary etiology of AP(53). The data are not definitive though and more studies are necessary.
Endoscopic retrograde cholangiopancreatography (ERCP)
ERCP with a possible therapeutic intervention should be considered if the results of imaging methods and/or laboratory tests (elevated FLT) suggest biliary etiology of AP(51). Early ERCP with sphincterotomy is required in patients with evident biliary obstruction or cholangitis and in patients in whom the course of AP is worsening despite full conservative treatment(2,8,66,67). The absence of macroscopic stones in CBD during ERCP does not exclude the presence of microlithiasis and in cases when the biliary etiology is strongly suspected sphincterotomy is justified(19). In case of proven biliary etiology the cholecystectomy should be carried out as soon as patient is fit for the surgery(66,68). Diagnostic value of ERCP in differential diagnostics of idiopathic acute pancreatitis is also in assessment of diagnosis of chronic pancreatitis, pancreas divisum, pancreatic and ampullary cancers or periampullary anomalies (e.g.choledochochele)(69).
Magnetic resonance cholangiopancreatography (MRCP)
MRCP is a rapidly evolving non-invasive method for imaging of biliary tree and its pathologies. In several recent studies its sensitivity reached 80-100%, a specificity of 91-100% and negative predictive value of 92-100% for the detection of choledocholithiasis(70-72). The future role of MRCP is yet to be defined, but with its increasing accuracy it may replace ERCP in diagnostic indications(53,72). In some studies MRCP appears to be nearly as sensitive as ERCP for the diagnosis of common bile duct stones(70).
Microscopic bile examination (MBE)
MBE is by some considered standard for the diagnosis of microlithiasis with overall sensitivity and specificity of 65% and 90%(13,73). It was suggested that finding of cholesterol crystals in bile during MBE is a marker of the presence of lithiasis(18,25,74). If available, MBE can be performed in cases with high clinical suspicion of biliary etiology of AP and negative results of less invasive methods(13).
According to study of Ros et al. the microscopic signs of microlithiasis were found in 67% of 51 patients with IAP(18). Presence of cholesterol monohydrate crystals can also predict successful cholelitholysis with UDCA therapy(18,23,75).
Associating EUS and MBE which can both be performed in one setting may be a highly accurate method for diagnosing or excluding of cholecystolithiasis (76). Combined EUS and stimulated biliary drainage has a high sensitivity of 92% and positive predictive value of 100%(76).
CONCLUSION
In the majority of patients the etiology of AP is apparent and can be fixed either by patient’s cooperation (alcohol cessation), medication, endoscopy or surgery but in important subgroup the diagnosis remains enigmatic after standard investigations (including laboratory blood tests and abdominal sonography). Close follow-up with repetition of laboratory tests and US and ERCP in case of suspected pathology of pancreatic or biliary ductal systems is one of the choices in these cases. Also new diagnostic methods and strategies are introduced to clinical practice with some of them (EUS, MRCP) becoming widely available and used. However, many sophisticated methods mentioned earlier (bile microscopy, sphincter of Oddi manometry, functional exocrine stimulation tests) which were proposed for revealing the underlying cause of the IAP are often invasive, unavailable in everyday clinical practice and with controversial value due to the literature. Therefore the definite diagnose is often not established and empiric cholecystectomy (CCX) is carried out after 2 attacks of idiopathic AP as recommended by most of the guidelines(2,11,13,43,55,77). CCX should cure patients with symptoms of microlithiasis, because biliary crystals usually form in the gallbladder and are rarely observed after the organ has been removed(24,36). Several studies suggest positive effect on recurrence of IAP after endoscopic sphincterotomy and UDCA therapy. Study of Testoni et al proved biliary sphincterotomy to be effective in 22 of 28 patients (78.6%) with additional pancreatic sphincterotomy to stop further attacks in 3 of remaining 6 patients. UDCA therapy was effective in 75% of patients with IRAP and normal radiological biliopancreatic findings suggesting the role of crystals(41). On the other hand other studies including our population-based retrospective study did not prove a positive effect of CCX on recurrence rates in patients with IRAP(78). Therefore, empiric CCX should be probably replaced with more intensive search for IRAP etiology in these patients.
Tento přehledový článek byl vytvořen ve spolupráci s týmem prof. Santhi S. Vegeho, MD v průběhu tříměsíční stáže MUDr. J. Trny a MUDr. V. Příbramské na Division of Gastroenterology, Mayo Clinic v Rochesteru, USA.
REFERRENCES
- 1. Gullo L, Migliori M, Olah A, et al. Acute pancreati tis in five European countries: Etiology and mortality. Pancreas 2002; 24: 223-227.
- 2. Alexis N, Lombard M, Raraty M, Ghaneh P, Smart HL, Gilmore I, Evans J, Hughes M, Garvey C, Sutton R, Neoptolemos JP. When is pancreatitis considered to be of biliary origin and what are the implications for management? Pancreatology 2007; 7: 131-141.
- 3. Ballinger AB, Barnes E, Alstead EM, Fairclough PD. Is intervention necessary after a first episode of acute idiopathic pancreatitis? Gut 1996; 38: 293-295.
- 4. Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med 1994; 330: 1198-1210.
- 5. Trapnell JE, Duncan EHL. Patterns of incidence in acute pancreatitis. Br Med J 1975; 2: 179-183.
- 6. Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: An algorithmic approach to identification and elimination of inciting factors. Gastroenterology 2001; 120: 708-717.
- 7. Clain JE, Pearson RK. Evidence-based approach to idiopathic pancreatitis. Curr Gastroent Rep 2002; 4: 128-134.
- 8. Kohut M, Nowak A, Nowakowska-Dulawa E, Kaczor R, Marek T. The frequency of bile duct crystals in patients with presumed biliary pancreatitis. Gastrointest Endosc 2001; 54: 37-41.
- 9. Bradley EL. A clinically based classification for acute pancreatitis. Arch Surg 1993; 128: 586-590.
- 10. Gregor JC, Ponich TP, Detsky AS. Should ERCP be routine after an episode of "idiopathic" pancreatitis? A cost-utility analysis. Gastrointest Endosc 1996; 44: 118-123.
- 11. Draganov P, Forsmark CE. "Idiopathic" pancreatitis. Gastroenterology 2005; 128: 756-763.
- 12. Choudari CP, Fogel EL, Sherman S, et al. Idiopathic pancreatitis: yield of ERCP correlated with patient age. Am J Gastroenterol 1998; 93: 1654A.
- 13. Levy M. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: Should we abandon the search or intensify our efforts? Gastrointest Endosc 2002; 55: 286-293.
- 14. Kloppel G, Maillet B. The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch 1992; 420: 1-4.
- 15. Lara LF, Levy MJ. Idiopathic recurrent acute pancreatitis. MedGenMed 2004; 6: 10.
- 16. Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastrol Hepatol 2007; 5: 75-79.
- 17. Conwell DL, Zuccaro G Jr, Vargo JJ, Morrow JB, Obuchowski N, Dumot JA, Trolli PA, Burton A, O’Laughlin C, Van Lente F. An endoscopic pancreatic function test with cholecystokinin-octapeptide for diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol 2003; 1: 189-194.
- 18. Ros E, Navarro S, Conxita B, Garcia-Puges A, Valderrama R. Occult microlithiasis in "idiopathic" acute pancreatitis: Prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology 1991; 101: 1701-1709.
- 19. Venneman NG, van Brummelen SE, van Berge- Henegouwen GP, van Erpecum KJ. Microlithiasis: an important cause of "idiopathic" acute pancreatitis? Ann Hepatol 2003; 2: 30-35.
- 20. Gerke H, Baillie J. Biliary microlithiasis: A neglected cause of recurrent pancreatitis and biliary colic? J Gastroenterol Hepatol 2005; 20: 499-501.
- 21. Venneman NG, Buskens E, Besselink MG, Stads S, Go P, Bosscha K, vanBerge-Henegouwen GP, van Erpecum KJ. Small gallstones are associated with increased risk of acute pancreatitis: Potential benefits of prophylactic cholecystectomy? Am J Gastroenterol 2005; 100: 2540-2550.
- 22. Diehl AK, Holleman DR Jr, Chapman JB, Schwesinger WH, Kurtin WE. Gallstone size and risk of pancreatitis. Arch Intern Med 1997; 157: 1674-1678.
- 23. Saraswat VA, Sharma BC, Agarwal DK, Kumar R, Negi TS, Tandon RK. Biliary microlithiasis in patients with idiopathic acute pancreatitis and unexplained biliary pain: Response to therapy. J Gastroenterol Hepatol 2004; 19: 1206-1211.
- 24. Sharma BC, Agarwal DK, Dhiman RK, Baijal SS, Choudhuri G, Saraswat VA. Bile lithogenicity and gallbladder emptying in patients with microlithiasis: effect of bile acid therapy. Gastroenterology 1998; 115: 124-128.
- 25. Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Eng J Med 1992; 326: 589-593.
- 26. Rashdan A, Fogel E, McHenry L Jr, Lehman G, Sherman S. Frequency of biliary crystals in patients with suspected sphincter of Oddi dysfunction. Gastroint Endosc 2003; 58: 875-878.
- 27. Welbourn CR, Beckly DE, Eyre-Brook IA. Endoscopic sphincterotomy without cholecystectomy for gall stone pancreatitis. Gut 1995; 37: 119-120.
- 28. Neoptolemos JP, Davidson BR, Winder AF. Role of duodenal bile crystals analysis in the investigation of "idiopathic" pancreatitis. Br J Surg 1988; 75: 450-453.
- 29. Andersen IB, Hojgaard L. Whither biliary sludge - do you exist? Gastroenterology 1991; 101: 1758-9.
- 30. Grau F, Almela P, Aparisi L, Bautista D, Pascual I, Pena A, Rodrigo JM. Usefulness of alanine and aspartate aminotransferases in the diagnosis of microlithiasis in idiopathic acute pancreatitis. Int J Pancreatol 1999; 25: 107-111.
- 31. Tenner S, Dubner H, Steinberg W. Predicting gallstone pancreatitis with laboratory parameters: A meta-analysis. Am J Gastroenterol 1994; 89: 1863- 1866.
- 32. Dholakia K, Pitchumoni CS, Agarwal N. How often are liver function tests normal in acute biliary pancreatitis? J Clin Gastroenterol 2004; 38: 81-83.
- 33. Dahan P, Andant C, Levy P, Amouyal G, Dumont M, Erlinger S, Sauvanet A, Belghiti J, Zins M, Vilgrain V, Bernades P. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut 1996; 38: 277-281.
- 34. Chebli JM, Gaburri PD, et al. "Idiopathic" acute pancreatitis due to biliary sludge: Prevention of relapses by endoscopic biliary sphincterotomy in high-risk patients. Am J Gastroenterol 2000; 95: 3008-3009.
- 35. Mayer AD, McMahon MJ, Benson EA, Axon AT. Operations upon the biliary tract in patients with acute pancreatitis: aims, indications and timing. Ann Royal Coll Surg Engl 1984; 66: 179-183.
- 36. Quallich LG, Stern MA, Rich M, Chey WD, Barnett JL, Elta GH. Bile duct crystals do not contribute to sphincter of Oddi dysfunction. Gastrointest Endosc 2002; 55: 163-166.
- 37. Guelrud M, et al. Idiopathic recurrent pancreatitis and hypercontractile sphincter of Oddi: treatment with endoscopic sphincterotomy and pancreatic duct dilation. Gastroenterology 1986; 90: 1443.
- 38. Sherman S, Troiano FP, Hawes RH, O’Connor KW, Lehman GA. Frequency of abnormal sphincter of Oddi manometry compared with clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol 1991; 86: 586-590.
- 39. Wehrmann T, Wiemer K, Lembcke B, Caspary WF, Jung M. Do patients with sphincter of Oddi dysfunction benefin from endoscopic sphincterotomy? A 5-year prospective trial. Eur J Gastroenterol Hepatol 1996; 8: 251-256.
- 40. Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. W J Gastroenterol 2008, 14: 1023-1026.
- 41. Testoni PA, Caporuscio S, Bagnolo F, Lella F. Idiopathic recurrent pancreatitis: Long-term results after ERCP, endoscopic sphincterotomy, or ursodeoxycholic acid treatment. Am J Gastroenterol 2000; 95: 1702-1707.
- 42. Steinberg WM. Should the sphincter of Oddi be measured in patients with idiopathic recurrent acute pancreatitis, ans should sphincterotomy be performed if the pressure is high? Pancreas 2003; 27: 118-121.
- 43. Steinberg WM, Barkin J, Bradley EL, DiMagno E, Layer P. Recurrent "idiopathic" acute pancreatitis: Should a laparoscopic cholecystectomy be the first procedure of choice? Pancreas 1996; 13: 329-334.
- 44. Warshaw AL. Pancreas divisum and pancreatitis. In: Berger HG, Warshaw AL, Russell RCG, Buchler M, Carr-Locke DL, Neoptolemos JP, Sarr MG eds. The pancreas. Oxford: Blackwell Science 1998: 364-374.
- 45. Delhaye M, Engelholm L, Cremer M. Pancreas divisum: congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology 1985; 89: 951-958.
- 46. Petrone MC, Arcidiacono PG, Testoni PA. Endoscopic ultrasonography for evaluating patients with recurrent pancreatitis. W J Gastroenterol 2008. 14: 1016-1022.
- 47. Steinberg WM, Chari ST, Forsmark CE, Sherman S, Reber HA, Bradley EL, DiMagno E. Management of acute idiopathic recurrent pancreatitis. Pancreas 2003; 27: 103-117.
- 48. McMahon MJ, Pickford IR. Biochemical prediction of gallstones early in an attack of acute pancreatitis. Lancet 1979; 2: 541-543.
- 49. Isogai M, Yamaguchi A, Hori A, Nakano S. Hepatic histopathological changes in biliary pancreatitis. Am J Gastroenterol 1995; 90: 449-454.
- 50. Lee CL, Wu CH, Chen TK, Yang SS, Huang CS. Prospective study of abdominal ultrasonography before laparoscopic cholecystectomy. J Clin Gastroenterol 1993; 1: 113-116.
- 51. Goodman AJ, Neoptolemos JP, Carr-Locke DL, Finlay DB, Fossard DP. Detection of gall stones after acute pancreatitis. Gut 1985; 26: 125-132.
- 52. Ammori BJ, Boreham B, Lewis P, Roberts SA. The biochemical detection of biliary etiology of acute pancreatitis on admission : A revisit in the modern era of biliary imaging. Pancreas 2003; 26: 32-35.
- 53. Sarosi G, Rege RV. Biliary pancreatitis. J Gastrointest Surg 2006; 1170-1178.
- 54. Pedersen OM, Nordgard K, Kvinnsland S. Value of sonography in obstructive jaundice. Limitations of bile duct caliber as an index of obstruction. Scand J Gastroenterol 1987; 22: 975-981.
- 55. Evans WB, Draganov P. Is empiric cholecystectomy a reasonable treatment option for idiopathic acute pancreatitis? Natur Clin Pract Gastroenterol Hepatol 2006; 3: 356-357.
- 56. Kurol M, Forsberg L. Ultrasonography in the diagnosis of cholecystitis. Acta Radiol Diagn Stockh 1984; 25: 379-383.
- 57. Balthazar EJ. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am 1989; 27: 19-37.
- 58. Gorry MC, Gabbaizedeh D, Furey W, Gates LK, Preston RA, Aston CE, Zhang Y, Ulrich CD, Ehrlich GD, Whitconb DC. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology 1997; 113: 1063-1068.
- 59. Choudari CP, Lehman GA, Sherman S. Pancreatitis and cystic fibrosis gene mutations. Gastroenterol Clin North Am 1999; 28: 543-549.
- 60. Dite P, Novotny I, Trna J, Sevcikova A. Autoimmune pancreatitis. Best Pract Res Clin Gastroenterol 2008; 22: 131-143.
- 61. Liu CL, Lo CM, Chan JKF, Poon RTP, Fan ST. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc 2000; 51: 28-32.
- 62. Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with "idiopathic" acute pancreatitis. Am J Med 2000; 109: 196-200.
- 63. Amouyal P, Amouyal G, Levy P, Tuzet S, Palazzo L, Vilgrain V. Diagnosis of choledocholithiasis by endoscopic ultrasonography. Gastroenterology 1994; 106: 1062-1067.
- 64. Scheiman JM, Carlos RC, Barnett JL, Elta GH, Nostrant TT, Chey WD, Francis IR, Nandi PS. Can endoscopic ultrasound or magnetic resonance cholangiography replace ERCP in patients with suspected biliary disease? A prospective trial and cost analysis. Am J Gastroenterol 2001; 96: 2900-2904.
- 65. Auberlin JM, Levoir D, Bouillot JT, Becheur H, Bloch F, Aouad K, et al. Endoscopic ultrasonography immediately prior to laparoscopic cholecystectomy: a prospective evaluation. Endoscopy 1996; 28: 667-673.
- 66. United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut 1998; 42 (Suppl 2): S1-S13.
- 67. Mergener K, Baillie J. Endoscopic treatment for acute biliary pancreatitis: when and in whom? Gastroenterol Clin North Am 1999; 28: 601-613.
- 68. Alimoglu O, Ozkan OV, Sahin M, Akcakaya A, Eryilmaz R, Bas G. Timing of cholecystectomy for acute biliary pancreatitis: Outcomes of cholecystectomy on first admission and after recurrent biliary pancreatitis. World J Surg 2003; 27: 256-259.
- 69. Liquory CL, Calleti G. An evaluation of endoscopic retrograde pancreatography (ERP) in chronic and relapsing acute pancreatitis. Endoscopy 1976; 8: 59-64.
- 70. Moon JH, Cho YD, Cha SW, et al. The detection of bile duct stones in suspected biliary pancreatitis: comparison of MRCP, ERCP and intraductal US. Am J Gastroenterol 2005; 100: 1051-1057.
- 71. Bearcraft PW, Lomas DJ. Magnetic resonance cholangiography. Gut 1997; 41: 135-137.
- 72. Hallal A, Amortegui J, Jeroukhimov I, Casillas J, Schulman C, Manning R, et al. Magnetic resonance cholangiopancreatography accurately detects common bile duct stones in resolving gallstone pancreatitis. J Am Coll Surg 2005; 200: 869-875.
- 73. Buscail L, Escourrou J, Delvaux M, Guimbaud R, Nicolet T, Frexinos J, Ribet A. Microscopic examination of bile directly collected during endoscopic cannulation of the papilla. Utility in patients with suspected microlithiasis. Dig Dis Sci 1992; 37: 116-120.
- 74. Ramond MJ, Dumont M, Belghiti J, Erlinger S. Sensitivity and specificity of microscopic examination of gallbladder bile for gallstone recognition and identification. Gastroenterology 1988; 95: 1339-1343.
- 75. Ros E, Navarro S, Fernandez I, Reixach M, Ribo JM, Rodes J. Utility of biliary microscopy for the prediction of the chemical composition of gallstones and the outcome of dissolution therapy with ursodeoxycholic acid. Gastroenterology 1986; 91: 703-712.
- 76. Dill JE, Hill S, Callis J, Berkhouse L, Evans P, Martin D, Palmer ST. Combined endoscopic ultrasound and stimulated biliary drainage in cholecystitis and microlithiasis - diagnoses and outcomes. Endoscopy 1995; 27: 424-427.
- 77. Shaffer EA. Gallbladder sludge: what is its clinical significance? Curr Gastroenterol Rep 2001; 3: 166-173.
- 78. Trna J, Vege SS, Pribramska V, Chari ST, Kamath PS. Recurrence of acute pancreatitis after cholecystectomy: A population-based study. Pancreatology 2009; 9: 433 (Abstract).
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users