Diagnosis and therapy of anaemia in patients with gastrointestinal tract diseases
Jaroslav Čermák Orcid.org 1
+ Affiliation
Summary
Anemia represents the most frequent hematological disorder in the world. In patiens with inflammatory bowel disease (IBD) several factors may contribute to development of anemia. Increased iron loss from the damaged intestinum surface is the most frequent cause of iron deficiency together with decreased rate of iron absorption from the gut. Simultaneously, the mechanisms involved in development of anemia of chronic disease (ACD) are also present in IBD. Increased level of inflammatory cytokines (IL-1, IL-6) stimulates secretion of hepcidin, a regulatory hormone that leads to retention of iron in the stores and blocks iron release in circulation for the need of erythropoiesis. A combination of serum ferritin level and transferin saturation represents the most effective laboratory tool for diagnosis of iron deficiency. A combination of several parameters (eg. serum ferritin, circulating transferrin receptor, serum hepcidin) is usually necessary for an exact diagnosis of iron deficiency in diseases with combined disorder of iron metabolism. Iron deficiency may be corrected with administration of mediactions containing iron salts. Parenteral administration of iron is indicated in patients with altered iron absorption from gut, new drugs with bioavailability due to relatively random and gradual release of even high content of iron in molecule (eg. ferric carboxymaltose) may effectively correct iron depletion.
Keywords
anemia, intestinal disease, diagnosis, hepcidin, treatment, iron deficiencyIntroduction
Anaemia is the most common haematological disorder and globally it represents the most frequent disease in the world. The leading cause of anaemia is iron deficiency; incidence of iron deficiency anaemia in our region is about 5 % in males and 8–20 % in females, however, in developing countries the reported incidence is up to 30–70 % of the population. A lack of iron is in a majority of cases absolute, i.e. iron stores within the body are reduced due to its insufficient supply or increased loss. A relative lack of iron available for erythropoiesis due to its retention in macrophages is the leading mechanism involved in anaemia in chronic diseases. The above mentioned mechanisms may combine in some diseases, eg. in patients with inflammation of the small or large intestine.
Iron metabolism and its regulation
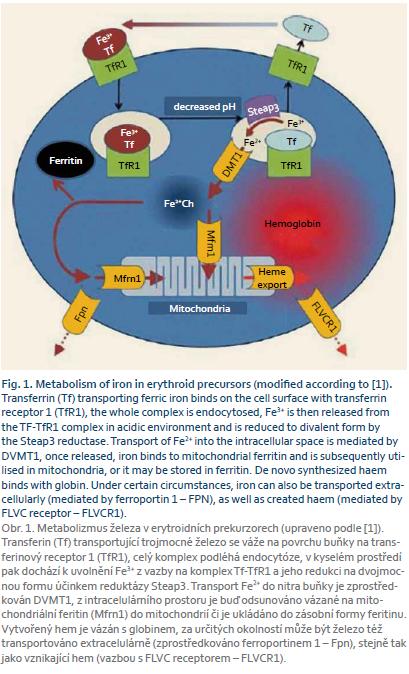
Iron absorbed from the gastrointestinal tract is transported into the target tissues predominantly bound in trivalent form to transferrin. Transferrin binds on the cell surface with the transferrin receptor and the whole complex is endocytosed. A drop in pH in the endocytic vesicle leads to the release of iron, which is reduced by Steap3 reductase to the divalent form and subsequently transported into the cell through DVMT1 (transporter for divalent iron, which mediates also the transport of iron from the intestine across the membrane of the intestinal epithelium). Once released into intracellular space, iron binds to mitochondrial ferritin and is subsequently utilised in mitochondria, or it may be stored in ferritin [1]. The metabolism of iron in erythroid precursors is shown in figure 1.
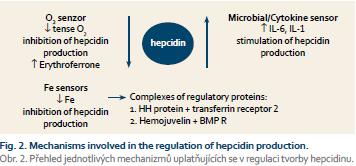
In 2000, hepcidin, a polypeptide synthesized in the liver that plays a key role in iron metabolism control, was discovered. The role of hepcidin is the degradation of ferroportin-1, which mediates the transfer of iron extracellularly; therefore the consequence of increased levels of hepcidin is a reduced output of iron from the cell. Many factors are involved in the control of hepcidin production, including hereditary haemochromatosis protein (HH or HFE protein) that binds in the cell to the transferrin receptor competitively with iron released from transferrin; free unbound HFE protein binds with transferrin receptor 2, and this complex induces formation of hepcidin through the activation of the bone morphogenic protein (BMP) receptor and its signalling pathways (SMAD). Recent findings suggest that the direct stimulation of the BMP receptor activity through BMP6 is obviously more important for control of iron metabolism and that BMP6 level is dependent on the concentration of iron in parenchymatous organs, especially in the liver. Additional regulatory proteins simulating BMP receptor activity are haemojuvelin and matriptase-2 (MT-2 or TM-PRSS6). Concordance of these regulatory mechanisms enables a delicate con- trol of the iron output from the monocyte-macrophage system into the circulation and secondarily of iron resorption from the gut in relation to in- creasing intracellular iron level. Similarly, the production of hepcidin is stimulated by haemojuvelin as well as by increased cytokine levels (IL-1, IL-6) in inflammation; this mechanism is the fundamental moment in the pathogenesis of anaemia in a chronic disease. Anaemia with hypoxia and accelerated erythropoiesis reduces production of hepcidin; the aim is to increase the output of iron into the circulation in order to move it into a functional pool in the bone marrow. It has been recently shown that this effect was mediated by a protein named erythroferrone [2], whose secretion was stimulated by enhanced endogenous erythropoietin (EPO) levels. This mechanism participates in the development of iron overload in some anaemias associated with escalated ineffective erythropoiesis (thalassemia, myelodysplastic syndrome) (Fig. 2).
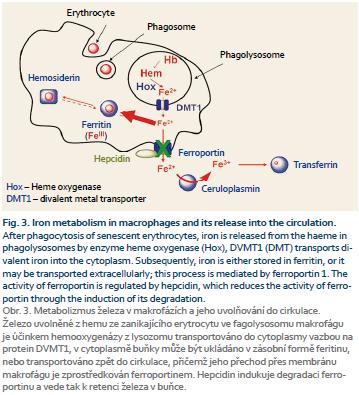
Figure 3 shows the metabolism of iron in the monocyte-macrophage system after phagocytosis of senescent erythrocytes. Iron is released from the heme in phagolysosomes by enzyme heme oxygenase (Hox), DVMT1 (DMT) transports divalent iron into the cytoplasm. Subsequently, iron is either stored in ferritin, or it may be transported extracellularly; this process is mediated by ferroportin 1. The activity of ferroportin is regulated by hepcidin, which reduces the activity of ferroportin through the induction of its degradation. Divalent iron is extracellularly oxidized in trivalent form by ceruloplasmin and it can be captured by transferrin again [3].
Causes of iron deficiency
The most common cause of iron deficiency is an excessive loss of iron from the body. In females, a most frequent source of bleeding is the urogenital tract. During menstruation the average amount of blood loss is 40–60 ml per cycle, which corresponds to about 16–25 mg of iron. An increased daily resorption of iron from the gut represents the first physiological regulatory mechanism for prevention of the development of iron deficiency in the blood. In menstruating females daily iron absorption from the gut is increased from 7–10 % to 20–25 %. When the blood loss exceeds 70–80 ml per cycle, the body is not able to compensate the loss of iron through an increased resorption and if the content of iron in food is not sufficiently increased (from normal 10–15 mg per day to about 18–20 mg), an iron deficiency may develop. Both benign and malignant tumours of the uterus can cause severe bleeding. Nephrolithiasis, ureteral stones and inflammatory affections of the kidneys and urinary tract are a less frequent, but non-negligible cause of chronic loss of blood and iron.
Gastrointestinal tract bleeding is another frequent cause of iron deficiency affecting men as well as women. From the wide range of disorders of the gut, we must highlight especially peptic ulcer, hernia with reflux oesophagitis, oesophageal varices, haemorrhoids, diverticulosis, Crohn‘s disease, ulcerative colitis, but also benign or malignant tumours. One should note that gastrointestinal bleeding can be caused by some medicines – acetylsalicylic acid, glucocorticosteroids, NSAIDs, anticoagulants; rarely by, for example, potassium chloride. Patients with bleeding disorders may loose blood from the gut because of impaired function of platelets or clotting factors. The less common causes of iron deficiency include bleeding from the respiratory tract and artificial losses, e. g. during haemodialysis and as a consquence of repeated blood sampling. In every donor, blood collection is associated with a loss of approximately 150–200 mg of iron. An insufficient content of iron in food is relatively rare in regions where the composition of food is similar to ours. Besides a direct deficiency of iron in food, a reduced absorption of iron can also be caused by an excessive content of substances which inhibit iron resorption (phosphates, phytates, tannates, oxalates). In parasitic diseases iron deficiency may be caused by a combination of impaired absorption with gastrointestinal bleeding. The consumption of iron together with gastrointestinal bleeding may lead to iron deficiency caused by Helicobacter pylori infection.
Increased demands on the intake of iron are seen during pregnancy when the iron need increases up to 20–25 mg daily. The loss of iron during pregnancy is estimated to be 900–1,000 mg, and particularly in the last trimester, no or insufficient substitution of iron can lead to development of iron deficiency in more than 70 % of pregnant women. During breastfeeding, the loss of iron makes up for about 1 mg per day. An increased consumption of iron is typical for the period of growth. In a two-year-old child the intake of iron should be about 15 mg per day, in a menstruating woman at the age of 14 and 30 years the daily intake of iron should be about 18 mg.
Mechanisms involved in pathogenesis of anaemia in chronic inflammatory bowel diseases
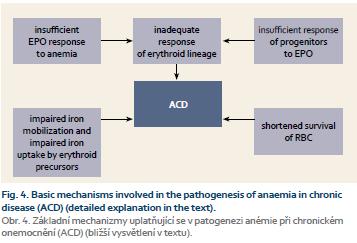
Anaemia associated with chronic inflammatory diseases of the gastrointestinal tract has the character of anaemia complicating chronic diseases. However, it is usually modified by other factors. As mentioned above, the crucial factor in pathogenesis of anemia in chronic diseases and tumours is the activation of the immune system which leads to a restricted supply of iron for the use by the pathogens or tumour cells where iron represents an essential growth factor. Simultaneously, these mechanisms are responsible for maintainence of an optimal iron concentration for the cytotoxic function of effector cells of the immune system. An increased secretion of cytokines (TNF-α, IL-6, IL-1, IL-4, IL-10, IFN-γ) by the cells of the activated immune system leads to the stimulation of the ferritin and hepcidin production. The result is a decrease in serum iron level due to its retention in the cells of the monocyte-macrophage system and secondarily an inhibition of iron absorption from the gut. Besides insufficient delivery of iron that can be utilized for erythropoiesis, the development of anaemia is also partly caused by an inhibitory effect of cytokines on the proliferation of erythroid precursors, by a reduced production of EPO and by shortened lifespan of erythrocytes due to alterations in the metabolism of proteins and lipids of erythrocyte membranes (Fig. 4) [4]. A decreased production of EPO is the main pathogenetic factor in anaemia associated with chronic renal insufficiency. In addition, in ACD a lack of inhibitory effect of EPO on the hepcidin production has been observed similarly as the lack of EPO stimulating effect on the formation of transferrin receptors for the cellular iron uptake.
A number of patients with chronic IBD also suffer from gastrointestinal bleeding from the damaged intestinal mucosa. Simultaneously, an inadequate intake of iron may be present as a manifestation of malabsorption, particularly in coeliac disease or Crohn‘s disease and after the resection of the stomach or intestines, where the resorption area is limited and the passage of food is accelerated. In the case of Helicobacter pylori infection, an additional effect of immune mechanisms on iron metabolism is suggested. Due to the multifactorial etiology of anaemia in chronic IBD, it is often necessary to use a combination of several laboratory tests to detect the presence and severity of iron deficiency and for an effective indication of substitution therapy (see below).
Diagnosis of iron deficiency
The stage of a prelatent iron deficiency with a gradual depletion of storage iron, but still with a sufficient supply for the needs of erythropoiesis, leads to development of mechanisms compensating an increased iron output from the stores. These mechanisms are represented by an increased resorption of iron from the gastrointestinal tract, by reduction of its output and a by decreased synthesis of ferritin, an iron storage protein. The content of stainable iron in the monocyte-macrophage system of the bone marrow is decreased; and serum ferritin drops below the lower limit of normal values in more than 50 % of patients. For clinical practice is critical an early diagnosis of latent iron deficiency, when iron stores are almost depleted and therefore its supply to the bone marrow is reduced. However, the degree of iron depletion is not so deep to be a limiting factor for erythropoiesis yet. Latent iron deficiency is characterized by serum ferritin level below 12 mg/l in more than 90 % of patients, the level of iron in serum is decreased, the total binding capacity of transferrin for iron is increased and transferrin saturation is < 16 %. Using a combination of serum ferritin level and transferrin saturation up to 95 % of patients with latent iron deficiency can be diagnosed. Thus, both the tests are essential for the diagnosis of iron deficiency. Simultaneously, the concentration of soluble circulating transferrin receptor in serum is increased (above 5–8 mg/l) as well as the concentration of free protoporphyrin in erythrocytes. In the bone marrow, the number of sideroblasts decreases from 50–60 % to < 30 %. At the stage of manifest iron deficiency, a lack of iron inhibits proliferation and anaemia develops due to the inability of erythropoiesis to compensate for a normal or slightly increased erythrocyte turnover. Manifest iron deficiency is characterised by hypochromic microcytic anaemia, serum ferritin reduced to < 5 µg/l, transferrin saturation < 10 %, and by a lack of storage iron in the bone marrow with < 10 % of sideroblasts. Along with the diagnosis of iron deficiency itself it is always necessary to investigate the cause of the decrease of iron stores. The investigation includes a repeated analysis of stools for blood loss, analysis of urine and urinary sediment, gynaecological examination and if necessary, even instrumental examination of GIT.
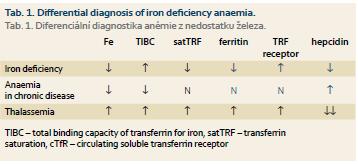
For the differential diagnosis we have to distinguish between early forms of iron deficiency and anaemia in chronic diseases or heterozygous forms of thalassemia. In patients with heterozygous β-thalassemia, significant microcytosis and hypochromia is present without significant anaemia, the number of erythrocyte count may be even slightly increased. Serum iron and ferritin are not decreased, characteristic changes are seen in haemoglobin electrophoresis and the serum hepcidin level is significantly decreased. Rarely, it is necessary to distinguish iron deficiency from congenital sideroblastic anaemia. In anaemia associated with chronic diseases serum iron level is decreased due to its retention in storage compartment in contrast with true iron deficiency, neither serum ferritin nor transferrin saturation are de- creased in ACD. The amount of circulating transferrin receptor (cTfR) is not in ACD increased and, unlike of iron deficiency, the level of serum hepcidin is elevated (Tab. 1).
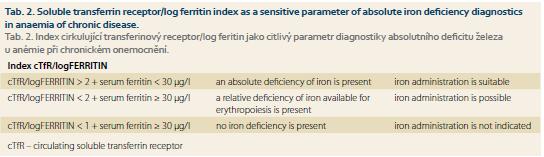
As noted above, in many chronic diseases, especially in chronic IBD, an increased loss of iron, its reduced resorption from gut and distribution disorders may combine. The reduced release of iron into the circulation represents in ACD a specific defence mechanism preventing the supply of iron as a growth factor for invading microorganisms or tumour cells, and therefore it is necessary to supply iron to the body only when a real iron deficiency has been documented. In ACD serum ferritin level is non-specifically increased due to the retention of iron in the monocyte-macrophage system, therefore, this parameter is of a little significance for the detection of the presence of real iron deficiency in ACD. A more sensitive indicator is the level of solubile circulating transferrin receptors; its increase reflects an increased synthesis of transferrin receptor in the cell in response to intracellular iron depletion (see the above-described regulatory mechanisms). Calculation of cTfR/log serum ferritin index represents even more sensitive parameter for detection of iron deficiency. Detection of elevated levels of hepcidin in serum can be an important tool for the diagnosis of predominance of relative functional iron deficiency caused by chronic disease itself. Unfortunately, no standardized commercial kit for detection of serum hepcidin level has been introduced yet. Therefore, the accurate diagnosis of the level of iron stores in chronic diseases is based on the use of a combination of several investigations [5]. The recommended combination of tests and their interpretation are presented in table 2.
Therapy of iron deficiency
The first step necessary for an effective treatment of iron deficiency therapy is the elimination of its causes. The second principle is delivery of a sufficient dose of iron for a sufficiently long period. The optimal treatment of iron deficiency should lead to an increase in Hb of 2 g/l per day; for such an increase at least 50–60 mg of elemental iron has to be absorbed, which corresponds with a daily dose of at least 180–200 mg of elemental iron taken per os in preparation with optimal resorption from gut. Preparations with iron should be administered evenly during the day, and if possible on an empty stomach at least half an hour before meals or at least two hours after a meal. A number of substances in food may decrease the absorption of iron, while amino acids, ascorbic acid and citric acid as well as some sugars promote resorption of iron and this fact is utilised in some preparations containing iron in combination with these compounds. Certain medicines can interfere with the resorption of iron (antacids, H2-receptor antagonists, pancreatic lipase, penicillamine, etc.). The administration of combined medicines with iron (mostly with vitamin B12 and folic acid) is appropriate only in case when a combined deficiency of these substances has been documented or prophylactically during pregnancy. To evaluate the effectiveness of the treatment we first perform the analysis of Hb level, after the normalization of Hb level serum ferritin should be rechecked and the patient should continue with the therapy to replenish iron stores in the body to optimal values, which is 300–500 mg of storage iron; 1 µg/l of ferritin in serum corresponds to about 8 mg of storage iron.
In patients with chronic IBD, one of the etiological factors of anaemia is, due to damage to the intestinal mucosa, a reduced absorption of iron from the gastrointestinal tract. In these patients, parenteral administration of iron is indicated. The normal dose is 62.5 mg (Fe3+ complex with sodium gluconate) or 100 mg (Fe3+ sucrose complex) of elemental iron once per day. The disadvantage of these medicines is a relatively rapid release of iron from the complex in the circulation, which can reduce its utilisation due to limited proliferation caused by iron deficiency. A quick and uncontrolled release of iron into the circulation is also the most common cause of adverse effects associated with parenteral administration of iron. The complex with Fe3+ carboxymaltose provides a slow and steady release of even high doses of iron in the molecule, which is associated with the utilisation of more than 90 % of administered iron with minimal side effects and with the possibility of application once a week [6]. Changes in the metabolism of iron in gastrointestinal tract disorders are often a result of multiple pathogenetic factors, and, as mentioned above, the actual diagnosis of iron deficiency usually requires a combination of several tests. In general and for simplification, we can say that a decrease in serum ferritin below 30–40 mg/l, a decrease in transferrin saturation below 16–18 % and an increase in cTfR levels above 3 mg/l in patients with IBD and other disorders of gastrointestinal tract may be a warning sign of an emerging absolute iron deficiency.
Therapeutic intervention focused on the mechanisms of anaemia in chronic disease
Currently, there are ongoing studies trying to correct increased levels of hepcidin as an etiological factor involved in the development of ACD. The recombinant erythropoietin (rHuEPO) itself has, besides anaemia in chronic renal insufficiency, little effect on improvement of ACD; its combination with the administration of erythroferrone that mediates the blocking effect of EPO on the production of hepcidin might be beneficial. Other substances investigated can be divided into groups of antibodies against hepcidin or against hepcidin production stimulatory mechanisms (antibodies against the IL-6 receptor), into a group of substances which interfere with the signalling pathways stimulating the production of mRNA for hepcidin (the signalling pathway of BMP – a bone morphogenic protein), and finally into a group of hepcidin-binding substances (some synthetic oligonucleotides) [7].
The author declares he has no potential conflicts of interest concerning drugs, products, or services used in the study.
Autor deklaruje, že v souvislosti s předmětem studie nemá žádné komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Submitted/Doručeno: 11. 8. 2015
Accepted/Přijato: 20. 8. 2015
Ass. prof. Jaroslav Čermák, M.D., Ph.D.
Institute of Hematology and Blood Transfusion
U nemocnice 2094/1
128 20 Praha 2
cermak@uhkt.cz
Literature
1. Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med 2012; 2 (5): doi: 10.1101/cshperspect.a011668.
2. Kautz L, Jung G, Valore E et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 2014; 46 (7): 678–684. doi: 10.1038/ng.2996.
3. Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest 2004; 113 (9): 1251–1253.
4. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352 (10): 1011–1023.
5. Cullis JO. Diagnosis and management of anemia of chronic disease: current status. Br J Haematol 2011; 154 (3): 289–300. doi: 10.1111/j.1365-2141.2011.087 41.x.
6. Geisser P. The pharmacology and safety profile of ferric carboxymaltose (Ferinject®): structure/reactivity relationships of iron preparations. Port J Nephrol Hypert 2009; 23 (1): 11–16.
7. Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematology Am Soc Hematol Educ Program 2013; 2013: 1–8. doi: 10.1182/asheducation-2013.1.1.