Novel developments in endoscopy of the proximal GIT Oliver Pech (Germany) – Gastro Update Europe 2018, Prague
Guido Tytgat1
+ Affiliation
Surveillance recommendations, according to European Society of Gastrointestinal Endoscopy guidelines, vary according to the length of the Barrett segment. Surveillance varies from: none for < 10 mm; every 5 year for < 3 cm; every 3 year for < 10 cm, and surveillance in a Barrett expert center if > 10 cm. Difference in expertise between a community hospital and an expert center was analysed in a retrospective multi-centric study involving 198 patients with high-grade dysplasia/adenocarcinoma. Detection of a visible lesion occurred respectively in 60 vs. 90%. In 40% neoplasia was only detected on non-targeted biopsies in community hospitals but was endoscopically detected in 75% by experts.
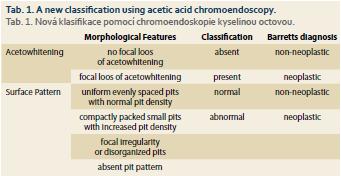
A new classification using acetic acid chromoendoscopy (PREDICT) has been presented (tab. 1) [1].
Acetic acid staining using this classification is easy to learn and can improve diagnostic performance in detecting Barrett neoplasia.
A model was developed to determine the risk of malignant progression of Barrett esophagus.The scoring system involved: Barrett length (1 point for each cm), male gender (9 points), smoking (5 points), low-grade dysplasia at baseline (11 points). The model was evaluated retrospectively in a longitudinal follow-up study involving 2,697 Barrett patients. The primary outcome parameter was the development of high-grade dysplasia/early adenocarcinoma during a median 5.9 year follow-up period. In low risk Group (0–10 points) progression risk was 0.13 %/year. In the intermediate risk Group (11–20 points) progression risk was 0.73 %/year. In the high risk Group (> 20 points) progression risk was 2.1 %/year.
Treatment op post-operative esophageal leakage can be: conservative (watch and wait, nil per os, tube feeding), per-endoscopic (OTS-clip, stent, endovac sponge therapy), surgical (anastomosis redo). A recent prospective evaluation of endoscopic vacuum therapy was carried out in 52 patients of which 75% had post-surgical leakage. First line therapy was endoscopic vacuum therapy with intraluminal or intracavitary sponge placement with 2 changes/w and with 100–125 mmHg negative pressure. Between 1–25 (~6) sponges were used. The defect healed in 94% of those only treated with sponges. Minor (dislocation, bleeding) and major (fatal bleeding) complications occured in respectively 31% and 4% of the patients. In-hospital mortality (bleeding, multi-organ failure, pneumonia) was 10%. Comparative studies are needed to find out which treatment modality is preferable for each anatomical presentation.
Standard criteria for endoscopic resection of early gastric cancer are: mucosal, G1-2, L0, V0, up to 20 mm. Novel expanded criteria are: submucosal sm1/< 500 microm or G3, or > 20 mm or < 30 mm in ulcerated lesions. Gradually ESD (endoscopic submucosal dissection) data from Europe is also becoming available as illustrated by a recent study involving 179 patients with 191 ESDs. ESD was incomplete because of ‘non-lifting’ in 5%. En-bloc resection and RO-resection were respectively 92 and 76%. Major complications (bleeding, perforation) occurred in 8% and procedure-related mortality was 1%. According to standard and expanded criteria, local recurrence was seen in 0 vs. 5%, metachronous lesions in 15 vs. 7%, need for surgery in 0 vs. 7% and total death/cancer death in (13/0 vs. 18/0%). Beyond doubt ESD for early gastric cancer will continue to expand in Europe in parallel with more refined methodology for lesion delineation etc., but for the time being such therapy will be restricted to dedicated expert centers because the patient volume is so far rather limited.
The risk of lymphnode metastasis in early gastric cancer is rather low, as again shown by a meta-analysis of 12 studies involving 9,798 gastrectomised patients. Lymphnode metastasis for standard vs. expanded criteria lesions were respectively 0.2 and 0.7%, for differentiated mucosal cancer < 3 cm with ulceration 0.57%, for differentiated mucosal cancer without ulceration 0.27% m, for undifferentiated mucosal cancer < 2 cm 2.6% and for differentiated submucosal cancer < 3 cm 2.5%. It is clear from such data that grade of differentiation and presence of ulceration are important prognostic features.
The role of Helicobacter pylori eradication (amoxicillin-clarithromycin-rabeprazole) after gastric ESD for early gastric cancer or high-grade dysplasia was evaluated in a large prospective placebo-controlled trial involving 470 patients, followed-up for a median of 5.9 year. In the eradication group (successful in 80%) and the placebo group, metachronous cancer occurred in respectively 7.2 vs. 13.4% and improvement of gastric mucosal atrophy in 48.4 vs. 15%. Thus the risk for metachronous gastric cancer after successful eradication dropped to an HR of o.32. After ESD, but also after partial gastrectomy for more advanced cancer, Helicobacter pylori eradication should be carried out.
Prof. Guido Tytgat, MD, PhD
Department of Gastroenterology and Hepatology
Academic Medical Center
Meibergdreef 9
1105 AZ Amsterdam
The Netherlands
g.n.tytgat@amc.uva.nl
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Kandiah K, Chedgy FJ, Subramaniam S et al. International development and validation of a classification system for the identification of Barrett‘s neoplasia using acetic acid chromoendoscopy: the Portsmouth acetic acid classification (PREDICT). Gut 2017; 67 (12): 2085–2091. doi: 10.1136/gutjnl-2017-314512