Intraoperative endoscopy is safe and helps to determine the resection extent in Crohn's disease
Dušan Podmanický1,2, Vladimír Štefanov3, Daniela Haruštiaková4, Zuzana Berecová5, Jan Kovács6, Zuzana Zelinková Orcid.org 7
+ Affiliation
Summary
Background: Intraoperative endoscopy can be of value in determining the extent of resection in Crohn’s disease (CD) patients, but data on its safety and usefulness in this setting are scarce. The aims of this study were first to analyse the safety of intraoperative endoscopy and then determine its impact on the extent of the resection. Patients and Methods: CD patients operated on in one centre between January 2015 and December 2016 were included. The differences in postoperative course and complications between the endoscopy group and the non-endoscopy group were analysed. In addition, the impact of intraoperative endoscopy findings on the extent of the resection was determined. Results: In total, 46 CD patients underwent surgery and 25 intraoperative endoscopies were performed in 20 patients. The endoscopy group had a significantly longer median hospital stay than the non-endoscopy group (respective medians of 6.5 vs. 5 days; p = 0.019). There were no significant differences between the two groups in other parameters. In 12 of the 20 patients in the endoscopy group, information provided by endoscopy led to change in the extent of resection. Conclusion: Intraoperative endoscopy is a safe and useful tool for tailoring the extent of surgery in CD patients.
Keywords
Crohn’s disease, intraoperative endoscopy, surgeryIntroduction
Crohn’s disease (CD) represents a chronic inflammatory condition affecting any part of the gastrointestinal tract. Despite great effort invested in the research of the disease pathogenesis over past years, the disease course remains unpredictable and only partially influenced by current therapeutic strategies [1]. Typically, during the disease course the dis-ease behaviour changes towards the stricturing and penetrating phenotype in nearly half of CD patients after 10 years following diagnosis [2]. This change in disease behaviour leads to complications, mainly intestinal stenosis and intra-abdominal abscess. In the case of fibrotic stenosis with obstructive symptoms, surgical resection or stricturoplasty remains the only therapeutic option thus far [3]. For intra-abdominal abscess complicating penetrating CD phenotype, percutaneous or surgical drainage is the first step followed by the escalation of anti-inflammatory therapy and in some cases a delayed planned resection [4].
The determination of the extent of the surgical resection remains a matter of debate, although it has become clear that no “clear margin” resection approach is beneficial [5] in terms of prevention of long-term post-surgical disease recurrence. Thus, currently in the majority of cases the preoperative assessment of the localisation and extent of the stenosis is done by means of cross-sectional imaging, preferentially magnetic resonance imaging (MRI), together with preoperative endoscopic assessment.
Both, MR enterography as well as ileo-colonoscopy represent a cumbersome procedure for the patient as both procedures require preparation with laxatives which can be difficult to complete for a patient with stricturing bowel disease. In addition, the procedures themselves are often limited in quality and incomplete in this specific patients’ population, although no specific data regarding this clinical setting are available. For the specific evaluation of the extent of small bowel disease, enteroscopy, especially double balloon enteroscopy, has been shown to be equally safe and effective in various conditions as compared to intraoperative enteroscopy [6,7] but the number of CD evaluated specifically with the intention of preoperative assessment was limited in this study. Finally, depending on the local health care structure and organisation, many CD patients with stricturing disease may consult the referral centre with surgical expertise in a clinical situation that requires immediate intervention. Therefore, preoperative assessment of the disease extent might sometimes not be possible or of low quality due to the mentioned reasons.
Furthermore, the sensitivity and specificity of preoperative assessment of disease extent by means of MR enterography varies across the studies [8] and is generally rather low when compared with the intraoperative judgement [9,10]. Especially in extensive disease, MR enterography seems to overestimate the length of the involved segment, however, underestimation may also occur in approximately one tenth of cases [9].
Thus, the thorough intraoperative assessment of the disease extent still represents an important part in the process of tailoring the type and extent of surgery in complicated CD. Intraoperative endoscopic assessment has been shown to be of additional value for intraoperative decision-making [11,12] but its safety has been questioned [13] mainly based on data on morbidity and mortality of intraoperative enteroscopies performed in obscure gastrointestinal bleeding conditions. Taking all factors into account, data on the safety of intraoperative endoscopic assessment of disease extent in the particular setting of CD are lacking and this procedure might be of additional value for the intraoperative decision-making on the extent of surgical resection.
Therefore, the aim of our study was firstly to determine the safety of intra-operative endoscopic assessment of disease activity and extent in CD patients undergoing surgery due to the stricturing or penetrating disease complication. Secondly, we evaluated its impact on the extent of the resection.
Patients and Methods
In a retrospective cohort study, all CD patients operated on in one centre between January 2015 and December 2016 were identified through diag-nosis coding used for insurance purposes. CD patients indicated for surgical intervention due to stricturing or penetrating disease complications or failure of medical therapy were eligible for further analysis.
The decision on the type and extent of the surgery was based on the multidisciplinary discussion of dedicated surgeons, gastroenterologists and radiologists. Intraoperative endoscopy was performed in case of unclear disease extent based on the preoperative MRI, when a recent MRI and/or endoscopy was not available or in case of previously detected extended small bowel disease (i.e. disease involving more than 30 cm of terminal ileum). The extent of the resection in each procedure was first evaluated by the surgeon, subsequently the extent was determined by the intraoperative endoscopy and the differences between the two evaluations were noted in the endoscopy report.
For the primary aim of the study, the duration of operation, hospital stay and complications such as anastomotic leak, abscess, readmission within 30 days were noted together with C reactive protein, procalcitonine and white blood cells during the days following the surgical intervention. With regards to the secondary aim of the study, for each endoscopic procedure, the impact on the intraoperative decision-making was noted.
The differences between the group of CD patients who underwent intraoperative endoscopy (endoscopy group) vs. the group without endoscopic assessment were analysed statistically.
Results
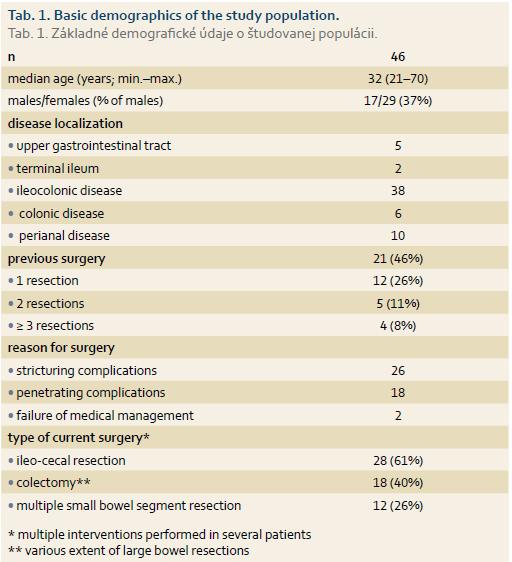
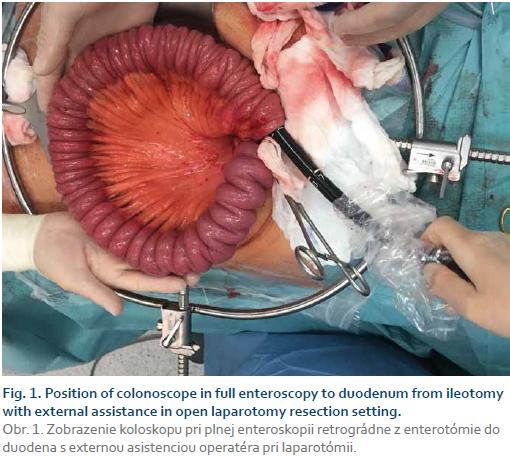
In total, 46 CD patients were included (basic demographics are shown in Tab. 1). There were 29 laparotomies (63%), 10 laparoscopic (21.7%) and 7 single port laparoscopic surgeries (15.3%) due to stricturing (26 patients) and penetrating (18 patients) disease complications or medical treatment failure (2 patients). Twenty-five intraoperative endoscopies were performed in 20 patients (14 enteroscopies, 3 gastroscopies – all three combined with enteroscopies, 11 colonoscopies). Out of 11 enteroscopies, there were 3 push enteroscopies using the oral route and 8 enteroscopies were performed using surgical enterotomy; in 3 cases the full length of the small bowel was examined (Fig. 1). Patients in endoscopy did not differ from the patients in non-endoscopy group with regards to basic demographics, more specifically, age, gender, surgical approach (laparoscopic vs. lap-arotomy), the number of previous surgical interventions and disease behaviour, i.e. stricturing vs. penetrating disease. A significantly lower proportion of patients with ileocecal disease localisation underwent intra-operative endoscopy (29% of all ileocecal localisation patients underwent endoscopy vs. 67% of all other localisations; p = 0.012), other disease phenotypes did not differ between the two groups.
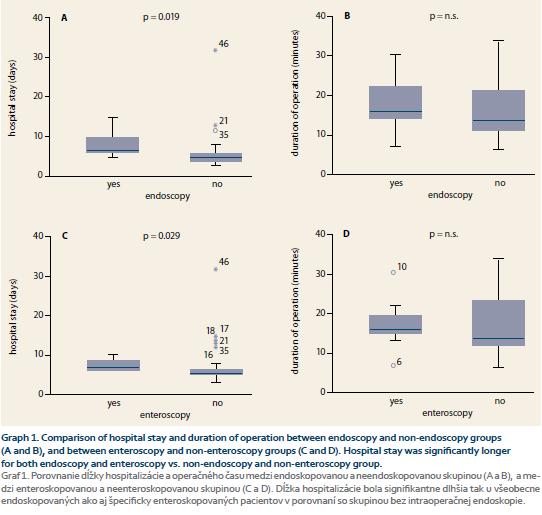
The endoscopy group had significantly longer median hospital stay compared with the group without endoscopy (respective medians of 6.5 vs. 5 days; p = 0.019; Graph 1A). There were no significant differences between the two groups with regards to the duration of the surgery (respective medians 160 vs. 135 min – endoscopy vs. no endoscopy group; p = n.s.; Graph 1B). C reactive protein, procalcitonine levels and white blood cells were numerically higher in the endoscopy group during the first 5 post-operative days but the difference was not statistically significant.
The subgroup of 11 patients who underwent intraoperative enteroscopy was analysed separately. The enteroscopy group had significantly longer hospital stay compared with the no enteroscopy group (respective medians 7 vs. 5 days; p = 0.029; Graph 1C). The duration of surgery did not differ between the enteroscopy and no enteroscopy groups (respective medians 160 vs. 135 min; p = n.s.; Graph 1D) and no differences were found between the C reactive protein, procalcitonine levels and white blood cells count between the two groups.
Complications occurred in one out of 20 patients in the endoscopy group (intra-abdominal abscess) and in one out of 26 patients in the non-endoscopy group (bleeding from the anastomosis); p = n.s. There was one readmission in the endoscopy group within 5 days after discharge; the reason was fever diagnosed as based on viral upper respiratory tract infection that was considered unrelated to the surgical procedure.
In 12 out of 20 patients (60%) who underwent intraoperative endoscopic assessment the information provided by endoscopy led to change in the extent of resection (5 reductions and 7 extensions of the segment to be resected) compared with the extent planned based on the cross-sectional imaging and the intraoperative judgement by surgeon.
Discussion
In this retrospective study we show that intraoperative endoscopic assessment of the extent of CD is safe in terms of short term postoperative complications. In addition, the informa-tion provided by intraoperative endoscopic assessment is crucial for the intraoperative decision-making on the extent of the surgery in 60% of patients.
Intraoperative endoscopy, more spe-cifically enteroscopy, was historically mainly used for the assessment of the origin of small intestinal bleeding in life threatening obscure gastrointestinal bleeding [13]. In these cohorts, the intraoperative endoscopy was related to a considerable morbidity and mortality of 5% and 17%, resp. [14]. Considering the clinical setting of a patient with gastrointestinal bleeding, this high mortality and morbidity might be inherent to the underlying condition rather than reflecting the risk profile of the procedure itself. Indeed, in our cohort, in line with two other previously published cohorts, this procedure seems to be safe.
In our cohort, the only negative outcome the intraoperative enteroscopy and endoscopy in general were associated with was longer hospital stay. Considering the design of this retrospective cohort, the difference between endoscopy and no endoscopy group in terms of hospital stay might be resulting from selection bias with a priori more complicated cases being selected for intraoperative endoscopic evaluation.
Another important aspect of intraoperative endoscopic assessment that was not dealt with in our study is the information that this assess-ment provides to treating gastroen-terologists for the postoperative medical management. Device-assisted enteroscopies are invasive procedures that can be replaced by intraoperative assessment in patients with an indication for surgery. Such detailed information about the extent of the disease is hardly available with current preoperative assessment and the findings at the intraoperative enteroscopy might shift the therapeutic decision about postoperative management. Considering the reassuring safety data of intraoperative enteroscopy based on this retrospective cohort, we believe a prospective assessment of this particular value of intraoperative enteroscopy is justified. Furthermore, a prospective setting would be able to determine the phenotype of CD patient who would benefit most from the intraoperative endoscopy which we were not able to provide with the present data.
In conclusion, intraoperative endoscopic assessment of the extent of CD does not bring additional complications and helps to better tailor the surgical decision in more than half of patients.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Submitted/Doručeno: 23. 1. 2017
Accepted/Přijato: 1. 2. 2017
Dusan Podmanicky, MD
Department of Surgery
St. Michael’s Hospital
Satinskeho 1
811 06 Bratislava
Slovak Republic
dusan.podmanicky@nsmas.sk
Literature
1. Mao EJ, Hazlewood GS, Kaplan GG et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2017; 45 (1): 3–13. doi: 10.1111/apt.13847.
2. Louis E, Collard A, Oger AF et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut 2001; 49 (6): 777–782.
3. Bettenworth D, Rieder F. Reversibility of stricturing Crohn’s disease-fact or fiction? Inflamm Bowel Dis 2016; 22 (1): 241–247. doi: 10.1097/MIB.0000000000000598.
4. Dignass A, Van AG, Lindsay JO et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis 2010; 4 (1): 28–62. doi: 10.1016/j.crohns.2009.12.002.
5. Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 2005; 11 (26): 3971–3979.
6. Kopacova M, Bures J, Vykouril L et al. Intraoperative enteroscopy: ten years’ experience at a single tertiary center. Surg Endosc 2007; 21 (7): 1111–1116.
7. Kopacova M, Bures J, Ferko A et al. Comparison of intraoperative enteroscopy and double-balloon enteroscopy for the diagnosis and treatment of Peutz-Jeghers syndrome. Surg Endosc 2010; 24 (8): 1904–1910. doi: 10.1007/s00464-009-0868-6.
8. Panes J, Bouzas R, Chaparro M et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011; 34 (2): 125–145. doi: 10.1111/j.1365-2036.2011.04710.x.
9. Brouquet A, Rangheard AS, Ifergan J et al. The accuracy of preoperative imaging in measuring the length of the ileocolic segment affected by Crohn’s disease: a prospective cohort study. Colorectal Dis 2016. In press. doi: 10.1111/codi. 13502.
10. Seastedt KP, Trencheva K, Michelassi F et al. Accuracy of CT enterography and magnetic resonance enterography imaging to detect lesions preoperatively in patients undergoing surgery for Crohn’s disease. Dis Colon Rectum 2014; 57 (12): 1364–1370. doi: 10.1097/DCR.0000000000000244.
11. Hotokezaka M, Jimi SI, Hidaka H et al. Role of intraoperative enteroscopy for surgical decision making with Crohn’s disease. Surg Endosc 2007; 21 (7): 1238–1242.
12. Smedh K, Olaison G, Nystrom PO et al. Intraoperative enteroscopy in Crohn’s disease. Br J Surg 1993; 80 (7): 897–900.
13. Pennazio M, Spada C, Eliakim R et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015; 47 (4): 352–376. doi: 10.1055/ s-0034-1391855.
14. Hartmann D, Schmidt H, Bolz G et al. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc 2005; 61 (7): 826–832.